Metformin alters therapeutic effects in the BALB/c tumor therapy model
- PMID: 34044797
- PMCID: PMC8161985
- DOI: 10.1186/s12885-021-08354-x
Metformin alters therapeutic effects in the BALB/c tumor therapy model
Abstract
Background: Despite considerable medical proceedings, cancer is still a leading cause of death. Major problems for tumor therapy are chemoresistance as well as toxic side effects. In recent years, the additional treatment with the antidiabetic drug metformin during chemotherapy showed promising results in some cases. The aim of this study was to develop an in vitro tumor therapy model in order to further investigate the potential of a combined chemotherapy with metformin.
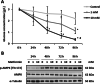
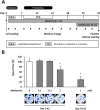
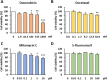
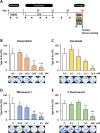
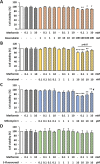
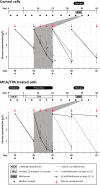
Methods: Cytotoxic effects of a combined treatment on BALB/c fibroblasts were proven by the resazurin assay. Based on the BALB/c cell transformation assay, the BALB/c tumor therapy model was established successfully with four different and widely used chemotherapeutics from different categories. Namely, Doxorubicin as a type-II isomerase inhibitor, Docetaxel as a spindle toxin, Mitomycin C as an alkylating agent and 5-Fluorouracil as an antimetabolite. Moreover, glucose consumption in the medium supernatant was measured and protein expressions were determined by Western Blotting.
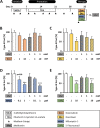
Results: Initial tests for the combined treatment with metformin indicated unexpected results as metformin could partly mitigate the cytotoxic effects of the chemotherapeutic agents. These results were further confirmed as metformin induced resistance to some of the drugs when applied simultaneously in the tumor therapy model. Mechanistically, an increased glucose consumption was observed in non-transformed cells as well as in the mixed population of malignant transformed cell foci and non-transformed monolayer cells, suggesting that metformin could also increase glucose consumption in transformed cells.
Conclusion: In conclusion, this study suggests a cautious use of metformin during chemotherapy. Moreover, the BALB/c tumor therapy model offers a potent tool for further mechanistic studies of drug-drug interactions during cancer therapy.
Keywords: Adjuvant; Cancer; Energy metabolism; In vitro model; Metformin.
Conflict of interest statement
The authors declare that they have no competing interests.
Figures
Similar articles
-
Butyrate and Metformin Affect Energy Metabolism Independently of the Metabolic Phenotype in the Tumor Therapy Model.Biomolecules. 2021 Dec 4;11(12):1831. doi: 10.3390/biom11121831. Biomolecules. 2021. PMID: 34944475 Free PMC article.
-
Efficacy of Metformin and Chemotherapeutic Agents on the Inhibition of Colony Formation and Shh/Gli1 Pathway: Metformin/Docetaxel Versus Metformin/5-Fluorouracil.Drug Res (Stuttg). 2021 Jan;71(1):17-25. doi: 10.1055/a-1248-9008. Epub 2020 Sep 28. Drug Res (Stuttg). 2021. PMID: 32987433
-
Technical modification of the Balb/c 3T3 cell transformation assay: the use of serum-reduced medium to optimise the practicability of the protocol.Altern Lab Anim. 2008 Dec;36(6):653-65. doi: 10.1177/026119290803600609. Altern Lab Anim. 2008. PMID: 19154092
-
Combinational strategies of metformin and chemotherapy in cancers.Cancer Chemother Pharmacol. 2016 Jul;78(1):13-26. doi: 10.1007/s00280-016-3037-3. Epub 2016 Apr 27. Cancer Chemother Pharmacol. 2016. PMID: 27118574 Review.
-
Metformin in breast cancer: preclinical and clinical evidence.Curr Probl Cancer. 2020 Feb;44(1):100488. doi: 10.1016/j.currproblcancer.2019.06.003. Epub 2019 Jun 20. Curr Probl Cancer. 2020. PMID: 31235186 Review.
Cited by
-
Antidiabetic Drugs in Breast Cancer Patients.Cancers (Basel). 2024 Jan 10;16(2):299. doi: 10.3390/cancers16020299. Cancers (Basel). 2024. PMID: 38254789 Free PMC article. Review.
-
Butyrate and Metformin Affect Energy Metabolism Independently of the Metabolic Phenotype in the Tumor Therapy Model.Biomolecules. 2021 Dec 4;11(12):1831. doi: 10.3390/biom11121831. Biomolecules. 2021. PMID: 34944475 Free PMC article.
References
-
- Cancer. https://www.who.int/news-room/fact-sheets/detail/cancer. Accessed 04 Nov 2020.
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

