Robust expression of LINE-1 retrotransposon encoded proteins in oral squamous cell carcinoma
- PMID: 34044801
- PMCID: PMC8161598
- DOI: 10.1186/s12885-021-08174-z
Robust expression of LINE-1 retrotransposon encoded proteins in oral squamous cell carcinoma
Abstract
Background: Oral Squamous Cell Carcinoma (OSCC) results from a series of genetic alteration in squamous cells. This particular type of cancer considers one of the most aggressive malignancies to control because of its frequent local invasions to the regional lymph node. Although several biomarkers have been reported, the key marker used to predict the behavior of the disease is largely unknown. Here we report Long INterpersed Element-1 (LINE1 or L1) retrotransposon activity in post-operative oral cancer samples. L1 is the only active retrotransposon occupying around 17% of the human genome with an estimated 500,000 copies. An active L1 encodes two proteins (L1ORF1p and L1ORF2p); both of which are critical in the process of retrotransposition. Several studies report that the L1 retrotransposon is highly active in many cancers. L1 activity is generally determined by assaying L1ORF1p because of its high expression and availability of the antibody. However, due to its lower expression and unavailability of a robust antibody, detection of L1ORF2p has been limited. L1ORF2p is the crucial protein in the process of retrotransposition as it provides endonuclease and reverse transcriptase (RT) activity.
Methods: Immunohistochemistry and Western blotting were performed on the post-operative oral cancer samples and murine tissues.
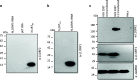
Results: Using in house novel antibodies against both the L1 proteins (L1ORF1p and L1ORF2p), we found L1 retrotransposon is extremely active in post-operative oral cancer tissues. Here, we report a novel human L1ORF2p antibody generated using an 80-amino-acid stretch from the RT domain, which is highly conserved among different species. The antibody detects significant L1ORF2p expression in human oral squamous cell carcinoma (OSCC) samples and murine germ tissues.
Conclusions: We report exceptionally high L1ORF1p and L1ORF2p expression in post-operative oral cancer samples. The novel L1ORF2p antibody reported in this study will serve as a useful tool to understand why L1 activity is deregulated in OSCC and how it contributes to the progression of this particular cancer. Cross-species reactivity of L1ORF2p antibody due to the conserved epitope will be useful to study the retrotransposon biology in mice and rat germ tissues.
Keywords: Cancer and L1 retrotransposon; L1 retrotransposon; L1ORF1p antibody; L1ORF2p antibody; OSCC and L1 retrotransposon.
Conflict of interest statement
The authors declare that they have no competing interests.
Figures





Similar articles
-
LINE-1 retrotransposon encoded ORF1p expression and promoter methylation in oral squamous cell carcinoma: a pilot study.Cancer Genet. 2020 Jun;244:21-29. doi: 10.1016/j.cancergen.2020.01.050. Epub 2020 Feb 10. Cancer Genet. 2020. PMID: 32088612
-
Human LINE-1 retrotransposition requires a metastable coiled coil and a positively charged N-terminus in L1ORF1p.Elife. 2018 Mar 22;7:e34960. doi: 10.7554/eLife.34960. Elife. 2018. PMID: 29565245 Free PMC article.
-
L1-ORF1p, a Smad4 interaction protein, promotes proliferation of HepG2 cells and tumorigenesis in mice.DNA Cell Biol. 2013 Sep;32(9):531-40. doi: 10.1089/dna.2013.2097. Epub 2013 Jul 17. DNA Cell Biol. 2013. PMID: 23863096
-
Prognostic and clinicopathological significance of PD-L1 overexpression in oral squamous cell carcinoma: A systematic review and comprehensive meta-analysis.Oral Oncol. 2020 Jul;106:104722. doi: 10.1016/j.oraloncology.2020.104722. Epub 2020 Apr 21. Oral Oncol. 2020. PMID: 32330687
-
Nucleic acid chaperone properties of ORF1p from the non-LTR retrotransposon, LINE-1.RNA Biol. 2010 Nov-Dec;7(6):706-11. doi: 10.4161/rna.7.6.13766. Epub 2010 Nov 1. RNA Biol. 2010. PMID: 21045547 Free PMC article. Review.
Cited by
-
Exploring transposable elements: new horizons in cancer diagnostics and therapeutics.Mob DNA. 2025 Jul 12;16(1):28. doi: 10.1186/s13100-025-00366-9. Mob DNA. 2025. PMID: 40646624 Free PMC article. Review.
-
Targeted detection of endogenous LINE-1 proteins and ORF2p interactions.Mob DNA. 2025 Feb 6;16(1):3. doi: 10.1186/s13100-024-00339-4. Mob DNA. 2025. PMID: 39915890 Free PMC article.
References
-
- Lander ES, Linton LM, Birren B, Nusbaum C, Zody MC, Baldwin J, Devon K, Dewar K, Doyle M, Fitz Hugh W, Funke R. Initial sequencing and analysis of the human genome.2001. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

