Extracellular vesicles: Roles and applications in drug-induced liver injury
- PMID: 34044913
- PMCID: PMC8982523
- DOI: 10.1016/bs.acc.2020.08.010
Extracellular vesicles: Roles and applications in drug-induced liver injury
Abstract
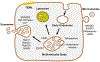
Extracellular vesicles (EV) are defined as nanosized particles, with a lipid bilayer, that are unable to replicate. There has been an exponential increase of research investigating these particles in a wide array of diseases and deleterious states (inflammation, oxidative stress, drug-induced liver injury) in large part due to increasing recognition of the functional capacity of EVs. Cells can package lipids, proteins, miRNAs, DNA, and RNA into EVs and send these discrete packages of molecular information to distant, recipient cells to alter the physiological state of that cell. EVs are innately heterogeneous as a result of the diverse molecular pathways that are used to generate them. However, this innate heterogeneity of EVs is amplified due to the diversity in isolation techniques and lack of standardized nomenclature in the literature making it unclear if one scientist's "exosome" is another scientist's "microvesicle." One goal of this chapter is to provide the contextual understanding of EV origin so one can discern between divergent nomenclature. Further, the chapter will explore the potential protective and harmful roles that EVs play in DILI, and the potential of EVs and their cargo as a biomarker. The use of EVs as a therapeutic as well as a vector for therapeutic delivery will be discussed.
Keywords: Acetaminophen; Biomarker; Drug-induced liver injury; Exosomes; Extracellular vesicles; Haptens; Idiosyncratic drug-induced liver injury; Microvesicle; Polycyclic aromatic hydrocarbons.
Copyright © 2021 Elsevier Inc. All rights reserved.
Conflict of interest statement
Conflict of interest The authors declare no conflict of interest.
Figures

Similar articles
-
Emerging role of extracellular vesicles in liver diseases.Am J Physiol Gastrointest Liver Physiol. 2019 Nov 1;317(5):G739-G749. doi: 10.1152/ajpgi.00183.2019. Epub 2019 Sep 23. Am J Physiol Gastrointest Liver Physiol. 2019. PMID: 31545919 Free PMC article. Review.
-
Extracellular vesicles in cardiovascular disease.Adv Clin Chem. 2021;103:47-95. doi: 10.1016/bs.acc.2020.08.006. Epub 2020 Oct 1. Adv Clin Chem. 2021. PMID: 34229852 Review.
-
Circulating extracellular vesicles as a potential source of new biomarkers of drug-induced liver injury.Toxicol Lett. 2014 Mar 21;225(3):401-6. doi: 10.1016/j.toxlet.2014.01.013. Epub 2014 Jan 21. Toxicol Lett. 2014. PMID: 24462978 Review.
-
Circulating Extracellular Vesicles As Biomarkers and Drug Delivery Vehicles in Cardiovascular Diseases.Biomolecules. 2021 Mar 5;11(3):388. doi: 10.3390/biom11030388. Biomolecules. 2021. PMID: 33808038 Free PMC article. Review.
-
Extracellular vesicles (EVs)' journey in recipient cells: from recognition to cargo release.J Zhejiang Univ Sci B. 2024 Aug 15;25(8):633-655. doi: 10.1631/jzus.B2300566. J Zhejiang Univ Sci B. 2024. PMID: 39155778 Free PMC article. Review.
Cited by
-
Orchestrated regulation of immune inflammation with cell therapy in pediatric acute liver injury.Front Immunol. 2023 Jun 22;14:1194588. doi: 10.3389/fimmu.2023.1194588. eCollection 2023. Front Immunol. 2023. PMID: 37426664 Free PMC article. Review.
-
The Role of Extracellular Vesicles in the Pathogenesis of Metabolic Dysfunction-Associated Steatotic Liver Disease and Other Liver Diseases.Int J Mol Sci. 2025 May 23;26(11):5033. doi: 10.3390/ijms26115033. Int J Mol Sci. 2025. PMID: 40507843 Free PMC article. Review.
-
Roles of Cofactors in Drug-Induced Liver Injury: Drug Metabolism and Beyond.Drug Metab Dispos. 2022 May;50(5):646-654. doi: 10.1124/dmd.121.000457. Epub 2022 Feb 27. Drug Metab Dispos. 2022. PMID: 35221288 Free PMC article. Review.
-
Effects of coenzyme Q10 and N-acetylcysteine on experimental poisoning by paracetamol in Wistar rats.PLoS One. 2023 Aug 22;18(8):e0290268. doi: 10.1371/journal.pone.0290268. eCollection 2023. PLoS One. 2023. PMID: 37607187 Free PMC article.
-
Venoms and Extracellular Vesicles: A New Frontier in Venom Biology.Toxins (Basel). 2025 Jan 14;17(1):36. doi: 10.3390/toxins17010036. Toxins (Basel). 2025. PMID: 39852989 Free PMC article. Review.
References
-
- Yanez-Mo M, Siljander PR, Andreu Z, Zavec AB, Borras FE, Buzas EI, Buzas K, Casal E, Cappello F, Carvalho J, Colas E, Silva A.C.-d., Fais S, Falcon-Perez JM, Ghobrial IM, Giebel B, Gimona M, Graner M, Gursel I, Gursel M, Heegaard NH, Hendrix A, Kierulf P, Kokubun K, Kosanovic M, Kralj-Iglic V, Kramer-Albers EM, Laitinen S, Lasser C, Lener T, Ligeti E, Line A, Lipps G, Llorente A, Lotvall J, Mancek-Keber M, Marcilla A, Mittelbrunn M, Nazarenko I, Nolte-’t Hoen EN, Nyman TA, O’Driscoll L, Olivan M, Oliveira C, Pallinger E, Del Portillo HA, Reventos J, Rigau M, Rohde E, Sammar M, Sanchez-Madrid F, Santarem N, Schallmoser K, Ostenfeld MS, Stoorvogel W, Stukelj R, Van der Grein SG, Vasconcelos MH, Wauben MH, De Wever O, Biological properties of extracellular vesicles and their physiological functions, J. Extracell. Vesicles 4 (2015) 27066. - PMC - PubMed
-
- Xu R, Rai A, Chen M, Suwakulsiri W, Greening DW, Simpson RJ, Extracellular vesicles in cancer—implications for future improvements in cancer care, Nat. Rev. Clin. Oncol. 15 (10) (2018) 617–638. - PubMed
-
- Johnsen KB, Gudbergsson JM, Andresen TL, Simonsen JB, What is the blood concentration of extracellular vesicles? Implications for the use of extracellular vesicles as blood-borne biomarkers of cancer, Biochim. Biophys. Acta, Rev. Cancer 1871 (1) (2019) 109–116. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

