Assessing vaccine introduction and uptake timelines in Gavi-supported countries: are introduction timelines accelerating across vaccine delivery platforms?
- PMID: 34045183
- PMCID: PMC8162093
- DOI: 10.1136/bmjgh-2021-005032
Assessing vaccine introduction and uptake timelines in Gavi-supported countries: are introduction timelines accelerating across vaccine delivery platforms?
Abstract
Background: Previous studies identified factors influencing regulatory approval to introduction timelines for individual vaccines. However, introduction and uptake timelines have not been comprehensively assessed across the portfolio of Gavi-supported vaccines.
Methods: We analysed median times between introduction milestones from vaccine licensure to country introduction and uptake across six vaccine-preventable diseases (VPDs), three delivery platforms and 69 Gavi-supported countries. Data were gathered from public, partner and manufacturer records. VPDs and prequalified vaccines analysed included Haemophilus influenzae type b (DTwP-HepB-Hib, pentavalent), pneumococcal disease (pneumococcal conjugate vaccine, PCV), rotavirus diarrhoea (rotavirus vaccine, RVV), cervical cancer (human papillomavirus vaccine, HPV), polio (inactivated polio vaccine, IPV) and meningococcal meningitis (meningococcal group A conjugate vaccine, MenA).
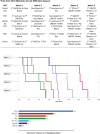
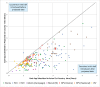
Results: Median time from first vaccine licensure to first Gavi-supported country introduction across VPDs at a 'global level' (Gavi-supported countries) was 5.4 years. Once licensed, MenA vaccines reached first introduction fastest (campaign=0.6 years; routine immunisation (RI)=1.7 years). Most introductions were delayed. Country uptake following first introduction was accelerated for more recently Gavi-supported RI vaccines compared with older ones.
Conclusion: Factors accelerating timelines across delivery platforms included rapid product prequalifications by WHO, strong initial recommendations by the WHO Strategic Advisory Group of Experts (SAGE) on Immunization, achieving target product profiles on first vaccine licensure within a VPD and completing several VPD milestones at a global level prior to licensure. Milestones required for introduction in Gavi-supported countries should start prior or in parallel to licensure to accelerate uptake of vaccines delivered through diverse delivery platforms.
Keywords: health policy; health systems; immunisation; vaccines.
© Author(s) (or their employer(s)) 2021. Re-use permitted under CC BY. Published by BMJ.
Conflict of interest statement
Competing interests: KL worked at IVAC at the time of analysis and at time of final revision and submission is employed by Gavi, the Vaccine Alliance. LP-D has received grants from Pfizer, Merck and GSK outside of submitted work.
Figures


Similar articles
-
The costs and effectiveness of large Phase III pre-licensure vaccine clinical trials.Expert Rev Vaccines. 2015;14(12):1543-8. doi: 10.1586/14760584.2015.1091733. Epub 2015 Sep 28. Expert Rev Vaccines. 2015. PMID: 26414015 Review.
-
New vaccine introductions in Africa before and during the decade of vaccines - Are we making progress?Vaccine. 2019 May 31;37(25):3290-3295. doi: 10.1016/j.vaccine.2019.05.002. Epub 2019 May 7. Vaccine. 2019. PMID: 31076160
-
Status of New Vaccine Introduction - Worldwide, September 2016.MMWR Morb Mortal Wkly Rep. 2016 Oct 21;65(41):1136-1140. doi: 10.15585/mmwr.mm6541a3. MMWR Morb Mortal Wkly Rep. 2016. PMID: 27764083
-
Role of Global Alliance for Vaccines and Immunization (GAVI) in Accelerating Inactivated Polio Vaccine Introduction.Indian Pediatr. 2016 Aug 7;53 Suppl 1:S57-S60. Indian Pediatr. 2016. PMID: 27771641
-
Shaping markets to benefit global health - A 15-year history and lessons learned from the pentavalent vaccine market.Vaccine X. 2019 Jul 18;2:100033. doi: 10.1016/j.jvacx.2019.100033. eCollection 2019 Aug 9. Vaccine X. 2019. PMID: 31384748 Free PMC article. Review.
Cited by
-
The potential impact of novel tuberculosis vaccine introduction on economic growth in low- and middle-income countries: A modeling study.PLoS Med. 2023 Jul 11;20(7):e1004252. doi: 10.1371/journal.pmed.1004252. eCollection 2023 Jul. PLoS Med. 2023. PMID: 37432972 Free PMC article.
-
Comparing research and development, launch, and scale up timelines of 18 vaccines: lessons learnt from COVID-19 and implications for other infectious diseases.BMJ Glob Health. 2023 Sep;8(9):e012855. doi: 10.1136/bmjgh-2023-012855. BMJ Glob Health. 2023. PMID: 37696544 Free PMC article. Review.
-
The role of vaccines in combating antimicrobial resistance (AMR) bacteria.Saudi J Biol Sci. 2021 Dec;28(12):7505-7510. doi: 10.1016/j.sjbs.2021.08.054. Epub 2021 Aug 24. Saudi J Biol Sci. 2021. PMID: 34867055 Free PMC article. Review.
-
Delayed completion of pneumococcal conjugate vaccination among children 4-48 months in rural Uganda: a socio-demographic inquiry.BMC Pediatr. 2025 Jan 14;25(1):29. doi: 10.1186/s12887-025-05388-z. BMC Pediatr. 2025. PMID: 39810121 Free PMC article.
-
COVID-19 vaccines and the pandemic: lessons learnt for other neglected diseases and future threats.BMJ Glob Health. 2023 Jun;8(6):e011883. doi: 10.1136/bmjgh-2023-011883. BMJ Glob Health. 2023. PMID: 37277196 Free PMC article. Review.
References
-
- World Health Organization . Global vaccine action plan: 2011–2020, 2013. Available: https://www.who.int/immunization/global_vaccine_action_plan/GVAP_doc_201... [Accessed 20 Nov 2017].
-
- Gavi, the Vaccine Alliance . Every child counts: the vaccine alliance progress report 2014, 2014. Available: http://www.gavi.org/results/gavi-progress-reports/ [Accessed 15 Nov 2017].
-
- World Health Organization . Assessment Report of the Global Vaccine Action Plan Strategic Advisory Group of Experts on Immunization 2015.
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
