Implementation of a complex intervention to improve hospital discharge: process evaluation of a cluster randomised controlled trial
- PMID: 34045217
- PMCID: PMC8162085
- DOI: 10.1136/bmjopen-2021-049872
Implementation of a complex intervention to improve hospital discharge: process evaluation of a cluster randomised controlled trial
Abstract
Objectives: To study the implementation of a cluster randomised controlled effectiveness-implementation hybrid trial testing the effectiveness of a medication review at hospital discharge combined with a communication stimulus between hospital physicians (HPs) and general practitioners (GPs) on rehospitalisation of multimorbid older patients.
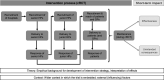
Design: Extension of Grant's mixed method process evaluation framework to trials with multilevel clustering.
Setting: General internal medicine wards in Swiss hospitals.
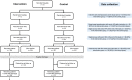
Participants: Convenience samples of 15 chief physicians (of 21 hospitals participating in the effectiveness trial), 60 (74) senior HPs, 65 (164) junior HPs and 187 (411) GPs.
Implementation strategy: Two-hour teaching sessions for senior HPs on a patient-centred, checklist-guided discharge routine.
Process evaluation components: Data collection on recruitment, delivery and response from chief physicians (semistructured interviews), senior HPs, junior HPs, GPs (surveys) and patients (via HPs). Quantitative data were summarised using descriptive statistics, and interviews analysed using thematic analysis.
Outcome measures: Intervention dose (quantitative), implementation fidelity (qualitative), feasibility and acceptability, facilitators and barriers, implementation support strategies.
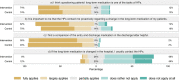
Results: Recruitment of hospitals was laborious but successful, with 21 hospitals recruited. Minimal workload and a perceived benefit for the clinic were crucial factors for participation. Intervention dose was high (95% of checklist activities carried out), but intervention fidelity was limited (discharge letters) or unknown (medication review). Recruitment and retention of patients was challenging, partly due to patient characteristics (old, frail) and the COVID-19 pandemic: Only 612 of the anticipated 2100 patients were recruited, and 31% were lost to follow-up within the first month after discharge. The intervention was deemed feasible and helpful by HPs, and the relevance of the topic appreciated by both HPs and GPs.
Conclusions: The results from this evaluation will support interpretation of the findings of the effectiveness study and may inform researchers and policy makers who aim at improving hospital discharge.
Trial registration number: ISRCTN18427377.
Keywords: general medicine (see internal medicine); public health; qualitative research; quality in health care.
© Author(s) (or their employer(s)) 2021. Re-use permitted under CC BY-NC. No commercial re-use. See rights and permissions. Published by BMJ.
Conflict of interest statement
Competing interests: None declared.
Figures

References
Publication types
MeSH terms
Associated data
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
