Silylation of Deoxynucleotide Analog Yields an Orally Available Drug with Antileukemia Effects
- PMID: 34045225
- PMCID: PMC9398096
- DOI: 10.1158/1535-7163.MCT-20-1125
Silylation of Deoxynucleotide Analog Yields an Orally Available Drug with Antileukemia Effects
Abstract
DNA methyltransferase inhibitors have improved the prognosis of myelodysplastic syndrome (MDS) and acute myeloid leukemia (AML). However, because these agents are easily degraded by cytidine deaminase (CDA), they must be administered intravenously or subcutaneously. Recently, two orally bioavailable DNA methyltransferase inhibitors, CC-486 and ASTX727, were approved. In previous work, we developed 5-O-trialkylsilylated decitabines that resist degradation by CDA. However, the effects of silylation of a deoxynucleotide analog and enzymatic cleavage of silylation have not been fully elucidated. Enteric administration of OR21 in a cynomolgus monkey model led to high plasma concentrations and hypomethylation, and in a mouse model, oral administration of enteric-coated OR21 led to high plasma concentrations. The drug became biologically active after release of decitabine (DAC) from OR21 following removal of the 5'-O-trisilylate substituent. Toxicities were tolerable and lower than those of DAC. Transcriptome and methylome analysis of MDS and AML cell lines revealed that OR21 increased expression of genes associated with tumor suppression, cell differentiation, and immune system processes by altering regional promoter methylation, indicating that these pathways play pivotal roles in the action of hypomethylating agents. OR21 induced cell differentiation via upregulation of the late cell differentiation drivers CEBPE and GATA-1 Thus, silylation of a deoxynucleotide analog can confer oral bioavailability without new toxicities. Both in vivo and in vitro, OR21 exerted antileukemia effects, and had a better safety profile than DAC. Together, our findings indicate that OR21 is a promising candidate drug for phase I study as an alternative to azacitidine or decitabine.
©2021 The Authors; Published by the American Association for Cancer Research.
Figures
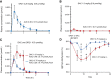
![Figure 1. Structure of OR21 and its effects on methylation. Structure of 5′-O-triethylsilyl-2′-deoxy-5-azacytidine (OR2100, OR21) and DAC (A). Agents with a log P value of +1.0 to +5.9 are expected to be absorbed after oral administration. Analysis of protein expression in MDS-L and HL60 cells treated with AZA, DAC, or OR21 (B). LINE-1 methylation analysis in MDS-L and SKM1 cells, as determined by bisulfite pyrosequencing (C). MDS-L and SKM1 cells were strongly hypermethylated (88.9% and 82.7%, respectively). Treatment with 100 nmol/L DAC or OR21 significantly decreased LINE-1 methylation levels in MDS-L [AZA, 88.8% (P = 0.867); DAC, 75.2% (P < 0.01); OR21, 74.2% (P < 0.01)] and SKM1 cells [AZA, 72.1% (P < 0.01); DAC, 58.4% (P < 0.01); OR21, 66.9% (P = 0.01)] (mean ± SD; n = 3). LINE-1 methylation analysis in AML cell lines treated with 500 nmol/L OR21. AML cell lines were also hypermethylated (KG1a, 89.1%; Kasumi-1, 90.7%; HL60, 83.7; THP-1, 69.0%; D). Treatment with OR21 tended to induce hypomethylation [KG1a, 82.4% (P = 0.091); Kasumi-1, 78.6% (P = 0.128); HL60, 76.0% (P = 0.089); THP-1, 64.6% (P = 0.243)] (mean ± SD; n = 3).](https://cdn.ncbi.nlm.nih.gov/pmc/blobs/6cec/9398096/3c995188ed1e/1412fig1.gif)

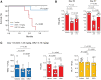
![Figure 5. SKM1 and HL60 xenograft mouse model experiments. Schedule of SKM1 cell xenograft experiments using BALB/c Rag-2/JAK3 double-deficient (BRJ) mice (A). Day 28: flow cytometry of hCD45-positive cells in mice treated with vehicle or OR21 (B). Kaplan–Meier survival curves showing cumulative survival of mice treated with vehicle or OR21 (n = 3 per cohort; C). Median overall survival time was 29 days (vehicle; P = 0.0246); NR = not reached. Statistical analysis was performed using the log-rank test. Schedule of HL60 cell xenograft experiments using BRJ mice (D). Day 37: flow cytometry of hCD45-positive cells in mice treated with vehicle (n = 5), DAC (n = 4), or OR21 (n = 4; E). Kaplan–Meier survival curves showing cumulative survival of mice treated with vehicle, DAC, or OR21 (F). Median overall survival times were 44 days (vehicle; n = 10), 46.5 days (DAC, n = 8, P = 0.164), and 49 days (OR21, n = 10, P = 0.005). Statistical analysis was performed using the log-rank test. DAC-treated mice were more likely to develop anemia than OR21-treated mice (G). Hemoglobin (Hb) levels were 17.5 g/dL (vehicle, n = 8), 15.6 g/dL (DAC, n = 6, P = 0.092), and 17.1 g/dL (OR21, n = 7, P = 1.0). LINE-1 methylation in bone marrow cells decreased after treatment with DAC or OR21 (n = 6 per cohort; H). Mice treated with a higher dose of DAC (2.0 mg/kg) did not live longer, whereas survival was prolonged in mice treated with OR21 (5.4 mg/kg) [median overall survival; vehicle, 48 days; DAC, 32 days (P = 0.040); OR21, 57 days (P < 0.001)] (I). HL60 xenograft mice exposed to late treatment with OR21 (day +28) had more CD11b-positive cells in PB on days +35 to +40, as determined by flow cytometry (n = 4 per cohort; vehicle, 43.3%; DAC, 75.7%, P = 0.229; OR21, 65.5%; P = 0.038; J).](https://cdn.ncbi.nlm.nih.gov/pmc/blobs/6cec/9398096/8cc927ac536d/1412fig5.gif)
Similar articles
-
Tracking Decitabine Incorporation into Malignant Myeloid Cell DNA in vitro and in vivo by LC-MS/MS with Enzymatic Digestion.Sci Rep. 2019 Mar 14;9(1):4558. doi: 10.1038/s41598-019-41070-y. Sci Rep. 2019. PMID: 30872721 Free PMC article.
-
Increased CDA expression/activity in males contributes to decreased cytidine analog half-life and likely contributes to worse outcomes with 5-azacytidine or decitabine therapy.Clin Cancer Res. 2013 Feb 15;19(4):938-48. doi: 10.1158/1078-0432.CCR-12-1722. Epub 2013 Jan 3. Clin Cancer Res. 2013. PMID: 23287564 Free PMC article.
-
The euphoria of hypomethylating agents in MDS and AML: is it justified?Best Pract Res Clin Haematol. 2013 Sep;26(3):275-8. doi: 10.1016/j.beha.2013.10.001. Epub 2013 Oct 15. Best Pract Res Clin Haematol. 2013. PMID: 24309530 Review.
-
Combination of a New Oral Demethylating Agent, OR2100, and Venetoclax for Treatment of Acute Myeloid Leukemia.Cancer Res Commun. 2023 Feb 21;3(2):297-308. doi: 10.1158/2767-9764.CRC-22-0259. eCollection 2023 Feb. Cancer Res Commun. 2023. PMID: 36860654 Free PMC article.
-
Clinical update on hypomethylating agents.Int J Hematol. 2019 Aug;110(2):161-169. doi: 10.1007/s12185-019-02651-9. Epub 2019 Apr 24. Int J Hematol. 2019. PMID: 31020568 Review.
Cited by
-
A Combination of Alectinib and DNA-Demethylating Agents Synergistically Inhibits Anaplastic-Lymphoma-Kinase-Positive Anaplastic Large-Cell Lymphoma Cell Proliferation.Cancers (Basel). 2023 Oct 21;15(20):5089. doi: 10.3390/cancers15205089. Cancers (Basel). 2023. PMID: 37894456 Free PMC article.
-
A novel anticancer quinolone, (R)-WAC-224, has anti-leukemia activities against acute myeloid leukemia.Invest New Drugs. 2023 Oct;41(5):751-760. doi: 10.1007/s10637-023-01393-0. Epub 2023 Sep 13. Invest New Drugs. 2023. PMID: 37702844
-
Treating Hematological Malignancies With OR-2100, an Orally Bioavailable Prodrug of Decitabine.Cancer Sci. 2025 Apr;116(4):853-861. doi: 10.1111/cas.16452. Epub 2025 Jan 21. Cancer Sci. 2025. PMID: 39837580 Free PMC article. Review.
-
Adult T-cell leukemia-lymphoma acquires resistance to DNA demethylating agents through dysregulation of enzymes involved in pyrimidine metabolism.Int J Cancer. 2022 Apr 1;150(7):1184-1197. doi: 10.1002/ijc.33901. Epub 2021 Dec 29. Int J Cancer. 2022. PMID: 34913485 Free PMC article.
-
DNA demethylating agents for chemoprevention of oncovirus-associated leukemogenesis.Leukemia. 2024 Jul;38(7):1613-1616. doi: 10.1038/s41375-024-02299-3. Epub 2024 Jun 5. Leukemia. 2024. PMID: 38839909 No abstract available.
References
-
- Tefferi A, Vardiman JW. Myelodysplastic syndromes. N Engl J Med 2009;361:1872–85. - PubMed
-
- Jordan CT. Unique molecular and cellular features of acute myelogenous leukemia stem cells. Leukemia 2002;16:559–62. - PubMed
-
- Esteller M. Relevance of DNA methylation in the management of cancer. Lancet Oncol 2003;4:351–8. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
Research Materials
Miscellaneous

