Adenovirus-induced Reactive Astrogliosis Exacerbates the Pathology of Parkinson's Disease
- PMID: 34045369
- PMCID: PMC8278136
- DOI: 10.5607/en21013
Adenovirus-induced Reactive Astrogliosis Exacerbates the Pathology of Parkinson's Disease
Abstract
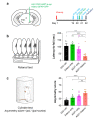
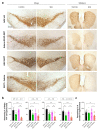
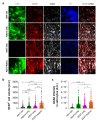
Parkinson's disease (PD) is the most prevalent neurodegenerative motor disorder. While PD has been attributed to dopaminergic neuronal death in substantia nigra pars compacta (SNpc), accumulating lines of evidence have suggested that reactive astrogliosis is critically involved in PD pathology. These pathological changes are associated with α-synuclein aggregation, which is more prone to be induced by an A53T mutation. Therefore, the overexpression of A53T-mutated α-synuclein (A53T-α-syn) has been utilized as a popular animal model of PD. However, this animal model only shows marginal-to-moderate extents of reactive astrogliosis and astrocytic α-synuclein accumulation, while these phenomena are prominent in human PD brains. Here we show that Adeno-GFAP-GFP virus injection into SNpc causes severe reactive astrogliosis and exacerbates the A53T-α-syn-mediated PD pathology. In particular, we demonstrate that AAV-CMV-A53T-α-syn injection, when combined with Adeno-GFAP-GFP, causes more significant loss of dopaminergic neuronal tyrosine hydroxylase level and gain of astrocytic GFAP and GABA levels. Moreover, the combination of AAV-CMV-A53T-α-syn and Adeno-GFAP-GFP causes an extensive astrocytic α-syn expression, just as in human PD brains. These results are in marked contrast to previous reports that AAV-CMV-A53T-α-syn alone causes α-syn expression mostly in neurons but rarely in astrocytes. Furthermore, the combination causes a severe PD-like motor dysfunction as assessed by rotarod and cylinder tests within three weeks from the virus injection, whereas Adeno-GFAP-GFP alone or AAV-CMV-A53T-α-syn alone does not. Our findings implicate that inducing reactive astrogliosis exacerbates PD-like pathologies and propose the virus combination as an advanced strategy for developing a new animal model of PD.
Keywords: Adenovirus infections; Alpha-synuclein; Mouse model; Parkinson’s disease; Reactive astrogliosis.
Figures

Similar articles
-
Cell senescence induced by toxic interaction between α-synuclein and iron precedes nigral dopaminergic neuron loss in a mouse model of Parkinson's disease.Acta Pharmacol Sin. 2024 Feb;45(2):268-281. doi: 10.1038/s41401-023-01153-z. Epub 2023 Sep 6. Acta Pharmacol Sin. 2024. PMID: 37674042 Free PMC article.
-
Rapid Induction of Dopaminergic Neuron Loss Accompanied by Lewy Body-Like Inclusions in A53T BAC-SNCA Transgenic Mice.Neurotherapeutics. 2022 Jan;19(1):289-304. doi: 10.1007/s13311-021-01169-5. Epub 2021 Dec 21. Neurotherapeutics. 2022. PMID: 34935120 Free PMC article.
-
Cx3cr1-deficiency exacerbates alpha-synuclein-A53T induced neuroinflammation and neurodegeneration in a mouse model of Parkinson's disease.Glia. 2018 Aug;66(8):1752-1762. doi: 10.1002/glia.23338. Epub 2018 Apr 6. Glia. 2018. PMID: 29624735
-
Adeno-Associated Virus Expression of α-Synuclein as a Tool to Model Parkinson's Disease: Current Understanding and Knowledge Gaps.Aging Dis. 2021 Jul 1;12(4):1120-1137. doi: 10.14336/AD.2021.0517. eCollection 2021 Jul. Aging Dis. 2021. PMID: 34221553 Free PMC article. Review.
-
Modeling Parkinson's Disease With the Alpha-Synuclein Protein.Front Pharmacol. 2020 Apr 23;11:356. doi: 10.3389/fphar.2020.00356. eCollection 2020. Front Pharmacol. 2020. PMID: 32390826 Free PMC article. Review.
Cited by
-
Overexpression of SIRT3 Suppresses Oxidative Stress-induced Neurotoxicity and Mitochondrial Dysfunction in Dopaminergic Neuronal Cells.Exp Neurobiol. 2021 Oct 31;30(5):341-355. doi: 10.5607/en21021. Exp Neurobiol. 2021. PMID: 34737239 Free PMC article.
-
Miniaturization of hiPSC-derived 3D neural cultures in stirred-tank bioreactors for parallelized preclinical assessment of rAAV.Front Bioeng Biotechnol. 2024 Apr 26;12:1379597. doi: 10.3389/fbioe.2024.1379597. eCollection 2024. Front Bioeng Biotechnol. 2024. PMID: 38737536 Free PMC article.
-
α-Synuclein-carrying astrocytic extracellular vesicles in Parkinson pathogenesis and diagnosis.Transl Neurodegener. 2023 Aug 25;12(1):40. doi: 10.1186/s40035-023-00372-y. Transl Neurodegener. 2023. PMID: 37620916 Free PMC article.
-
Longitudinal correlation of cerebrospinal fluid GFAP and the progression of cognition decline in different clinical subtypes of Parkinson's disease.Clin Transl Sci. 2024 Dec;17(12):e70111. doi: 10.1111/cts.70111. Clin Transl Sci. 2024. PMID: 39676304 Free PMC article.
-
Cerebrospinal fluid GFAP is a predictive biomarker for conversion to dementia and Alzheimer's disease-associated biomarkers alterations among de novo Parkinson's disease patients: a prospective cohort study.J Neuroinflammation. 2023 Jul 20;20(1):167. doi: 10.1186/s12974-023-02843-5. J Neuroinflammation. 2023. PMID: 37475029 Free PMC article.
References
-
- Gispert S, Ricciardi F, Kurz A, Azizov M, Hoepken HH, Becker D, Voos W, Leuner K, Müller WE, Kudin AP, Kunz WS, Zimmermann A, Roeper J, Wenzel D, Jendrach M, García-Arencíbia M, Fernández-Ruiz J, Huber L, Rohrer H, Barrera M, Reichert AS, Rüb U, Chen A, Nussbaum RL, Auburger G. Parkinson phenotype in aged PINK1-deficient mice is accompanied by progressive mitochondrial dysfunction in absence of neurodegeneration. PLoS One. 2009;4:e5777. doi: 10.1371/journal.pone.0005777. - DOI - PMC - PubMed
-
- Lee MK, Stirling W, Xu Y, Xu X, Qui D, Mandir AS, Dawson TM, Copeland NG, Jenkins NA, Price DL. Human alpha-synuclein-harboring familial Parkinson's disease-linked Ala-53 --> Thr mutation causes neurodegenerative disease with alpha-synuclein aggregation in transgenic mice. Proc Natl Acad Sci U S A. 2002;99:8968–8973. doi: 10.1073/pnas.132197599. - DOI - PMC - PubMed
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

