Neratinib plus trastuzumab is superior to pertuzumab plus trastuzumab in HER2-positive breast cancer xenograft models
- PMID: 34045483
- PMCID: PMC8159999
- DOI: 10.1038/s41523-021-00274-0
Neratinib plus trastuzumab is superior to pertuzumab plus trastuzumab in HER2-positive breast cancer xenograft models
Abstract
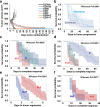
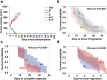
Lapatinib (L) plus trastuzumab (T), with endocrine therapy for estrogen receptor (ER)+ tumors, but without chemotherapy, yielded meaningful response in HER2+ breast cancer (BC) neoadjuvant trials. The irreversible/pan-HER inhibitor neratinib (N) has proven more potent than L. However, the efficacy of N+T in comparison to pertuzumab (P) + T or L + T (without chemotherapy) remains less studied. To address this, mice bearing HER2+ BT474-AZ (ER+) cell and BCM-3963 patient-derived BC xenografts were randomized to vehicle, N, T, P, N+T, or P+T, with simultaneous estrogen deprivation for BT474-AZ. Time to tumor regression/progression and incidence/time to complete response (CR) were determined. Changes in key HER pathway and proliferative markers were assessed by immunohistochemistry and western blot of short-term-treated tumors. In the BT474-AZ model, while all N, P, T, N + T, and P + T treated tumors regressed, N + T-treated tumors regressed faster than P, T, and P + T. Further, N + T was superior to N and T alone in accelerating CR. In the BCM-3963 model, which was refractory to T, P, and P + T, while N and N + T yielded 100% CR, N + T accelerated the CR compared to N. Ki67, phosphorylated (p) AKT, pS6, and pERK levels were largely inhibited by N and N + T, but not by T, P, or P + T. Phosphorylated HER receptor levels were also markedly inhibited by N and N + T, but not by P + T or L + T. Our findings establish the efficacy of combining N with T and support clinical testing to investigate the efficacy of N + T with or without chemotherapy in the neoadjuvant setting for HER2+ BC.
Conflict of interest statement
C.G.: Research grant from Ventana. M.G.: speaker bureau for Novartis, Eli Lilly, Pfizer, Celgene, Eisai, and AstraZeneca; accommodation and travel support from Roche and Pfizer. C.D.A.: consultant/advisory board member for Novartis, Eli Lilly, and Pfizer; Research grant from Novartis (to the Institution). M.F.R.: research support from GSK (to institution); consulting with Genentech, Novartis, Daiichi, and Macrogenics. I.D.: employee of Puma Biotechnology Inc. A.S.L.: employee of Puma Biotechnology Inc. C.K.O.: research funding from AstraZeneca and GlaxoSmithKline; advisory boards for Tolmar Pharmaceuticals, Genentech, and AstraZeneca; DMC for Eli Lilly; Stockholder of GeneTex. R.S.: research funding from AstraZeneca, GlaxoSmithKline, Puma Biotechnology Inc. (to the institution), and Gilead Sciences; past ad hoc advisory committee member for Eli Lilly; and consulting/advisory committee member for Macrogenics. The remaining authors declare no competing interests
Figures


References
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

