Effects of potent neutralizing antibodies from convalescent plasma in patients hospitalized for severe SARS-CoV-2 infection
- PMID: 34045486
- PMCID: PMC8160346
- DOI: 10.1038/s41467-021-23469-2
Effects of potent neutralizing antibodies from convalescent plasma in patients hospitalized for severe SARS-CoV-2 infection
Abstract
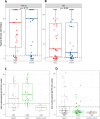
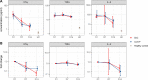
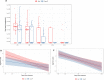
In a randomized clinical trial of 86 hospitalized COVID-19 patients comparing standard care to treatment with 300mL convalescent plasma containing high titers of neutralizing SARS-CoV-2 antibodies, no overall clinical benefit was observed. Using a comprehensive translational approach, we unravel the virological and immunological responses following treatment to disentangle which COVID-19 patients may benefit and should be the focus of future studies. Convalescent plasma is safe, does not improve survival, has no effect on the disease course, nor does plasma enhance viral clearance in the respiratory tract, influence SARS-CoV-2 antibody development or serum proinflammatory cytokines levels. Here, we show that the vast majority of patients already had potent neutralizing SARS-CoV-2 antibodies at hospital admission and with comparable titers to carefully selected plasma donors. This resulted in the decision to terminate the trial prematurely. Treatment with convalescent plasma should be studied early in the disease course or at least preceding autologous humoral response development.
Conflict of interest statement
The Netherlands Organisation for Health Research and Development (ZonMW, grant agreement 10150062010008, B.L.H.), the Erasmus foundation grant (B.J.A.R.), and the PPP Allowance (grant number LSHM20056, P.D.K.) made available by Health~Holland, Top Sector Life Sciences & Health, to stimulate public-private partnerships. The remaining authors declare no competing interests.
Figures





References
-
- Study assessing the safety, tolerability, pharmacokinetics, and immunogenicity of repeated subcutaneous doses of anti-spike (S) SARS-CoV-2 monoclonal antibodies (REGN10933+REGN10987) in adult volunteers as related to COVID-19, https://clinicaltrials.gov/ct2/show/study/NCT04519437?term=Regeneron+Pharmaceuticals&cond=%22Coronavirus+Infections%22&draw=2&rank=2#wrapper (accessed 20 October 2020).
-
- Safety, tolerability, and efficacy of anti-spike (S) SARS-CoV-2 monoclonal antibodies for the treatment of ambulatory adult patients with COVID-19, https://clinicaltrials.gov/ct2/show/NCT04425629?term=Regeneron+Pharmaceuticals&cond=%22Coronavirus+Infections%22&draw=2&rank=3 (accessed 20 October 2020).
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous

