Effects of danofloxacin dosing regimen on gastrointestinal pharmacokinetics and fecal microbiome in steers
- PMID: 34045586
- PMCID: PMC8160337
- DOI: 10.1038/s41598-021-90647-z
Effects of danofloxacin dosing regimen on gastrointestinal pharmacokinetics and fecal microbiome in steers
Abstract
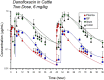
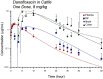
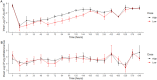
Fluoroquinolones are a class of antimicrobial commonly used in human medicine, and deemed critical by the World Health Organization. Nonetheless, two formulations are approved for the treatment of respiratory disease in beef cattle. The objective of this study was to determine the gastrointestinal pharmacokinetics and impact on enteric bacteria of cattle when receiving one of the two dosing regimens (high: 40 mg/kg SC once or low: 20 mg/kg IM q48hr) of danofloxacin, a commonly utilized synthetic fluoroquinolone in veterinary medicine. Danofloxacin was administered to 12 steers (age 7 months) fitted with intestinal ultrafiltration devices at two different dosing regimens to assess the gastrointestinal pharmacokinetics, the shifts in the gastrointestinal microbiome and the development of resistant bacterial isolates. Our results demonstrated high intestinal penetration of danofloxacin for both dosing groups, as well as, significant differences in MIC values for E. coli and Enterococcus between dosing groups at selected time points over a 38 day period. Danofloxacin treatment consistently resulted in the Euryarchaeota phyla decreasing over time, specifically due to a decrease in Methanobrevibacter. Although microbiome differences were minor between dosing groups, the low dose group had a higher number of isolates with MIC values high enough to cause clinically relevant resistance. This information would help guide veterinarians as to appropriate dosing schemes to minimize the spread of antimicrobial resistance.
Conflict of interest statement
The authors declare no competing interests.
Figures


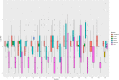
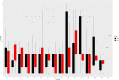

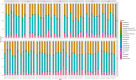
References
-
- Pfizer. Freedom of Information Summary Advocin 2011, NADA 141-207.
-
- U.S. Department of Health and Human Services. Guidance for Industry: Evaluating the Safety of Antimicrobial New Animal Drugs with Regard to Their Microbiological Effects on Bacteria of Human Health Concerns (2003). https://www.fda.gov/regulatory-information/search-fda-guidance-documents.... Accessed (2019)
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

