Long-Term Effects of Iron Chelating Agents on Ocular Function in Patients with Thalassemia Major
- PMID: 34045846
- PMCID: PMC8144174
- DOI: 10.2147/OPTH.S300974
Long-Term Effects of Iron Chelating Agents on Ocular Function in Patients with Thalassemia Major
Abstract
Background: The aim of this study is to evaluate eye structures and function in patients receiving iron chelating therapy and to assess whether a correlation exists between the onset of ocular alterations and the intake of iron chelating drugs.
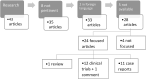
Methods: A prospective cohort study was performed. Eighty-eight patients, composed of children and adults with thalassemia major (TM) who are taking or had taken iron chelating drugs (deferoxamine, deferiprone or deferasirox), have been initially enrolled in the study. The final sample featured 80 patients, including 18 children and 62 adults. These subjects received an eye examination to evaluate intraocular pressure (IOP), best corrected visual acuity (BCVA), the presence of refractive defects, cornea, anterior chamber, lens, fundus oculi, visual field and mean retinal nerve fiber layer (RNFL) thickness. Logistic regression model analysis was performed in order to assess any correlation. In addition, a literature search regarding the relation between iron chelating drugs and ocular adverse events was carried out to compare the results obtained with the evidence in the literature.
Results: Logistic regression did not report a significant correlation between the intake of iron chelating drugs and the onset of anterior ocular segment alterations, lens opacities, retinal diseases, optical neuropathies, astigmatism, visual field and RNFL thickness defects. Logistic regression returned a statistically significant correlation between myopia and iron chelation therapy (p-value 0.04; OR 1.05) and also between presbyopia and total duration of therapy with deferoxamine (p-value 0.03; OR 1.21). Although intraocular pressure levels remained within the normal range, a significant correlation with the length of deferoxamine therapy has been found (p-value 0.002; association coefficient -0.12). A negative correlation between deferiprone and presbyopia has also been observed.
Conclusion: Iron chelation therapy is not associated with severe visual function alterations. Limitation of deferoxamine treatment can help prevent ocular complications. Deferiprone and/or deferasirox may be preferable, especially in patients over age 40 years.
Keywords: deferasirox; deferiprone; deferoxamine; iron chelation; ocular adverse effects.
© 2021 Nuzzi et al.
Conflict of interest statement
Prof. Dr. Antonio Giulio Piga reports grants from Novartis, grants from Apopharma, outside the submitted work. The authors declare that there are no conflicts of interest regarding the publication of this paper.
Figures


Similar articles
-
Interventions for improving adherence to iron chelation therapy in people with sickle cell disease or thalassaemia.Cochrane Database Syst Rev. 2023 Mar 6;3(3):CD012349. doi: 10.1002/14651858.CD012349.pub3. Cochrane Database Syst Rev. 2023. PMID: 36877640 Free PMC article. Review.
-
Phakic intraocular lenses for the treatment of refractive errors: an evidence-based analysis.Ont Health Technol Assess Ser. 2009;9(14):1-120. Epub 2009 Oct 1. Ont Health Technol Assess Ser. 2009. PMID: 23074518 Free PMC article.
-
Therapeutic efficacy of different iron chelators in Egyptian children with Beta Thalassemia with iron overload.Infect Disord Drug Targets. 2015;15(2):98-105. doi: 10.2174/1871526515666150724111721. Infect Disord Drug Targets. 2015. PMID: 26205801
-
Efficacy and safety of iron-chelation therapy with deferoxamine, deferiprone, and deferasirox for the treatment of iron-loaded patients with non-transfusion-dependent thalassemia syndromes.Drug Des Devel Ther. 2016 Jan 29;10:465-81. doi: 10.2147/DDDT.S79458. eCollection 2016. Drug Des Devel Ther. 2016. PMID: 26893541 Free PMC article. Review.
-
Differential effects of the type of iron chelator on the absolute number of hematopoietic peripheral progenitors in patients with β-thalassemia major.Haematologica. 2013 Apr;98(4):555-9. doi: 10.3324/haematol.2012.076240. Epub 2012 Dec 14. Haematologica. 2013. PMID: 23242593 Free PMC article.
Cited by
-
Ocular biometry, anterior chamber morphometry, and their relationship with serum ferritin levels in children with beta thalassemia major.Ther Adv Ophthalmol. 2023 Apr 20;15:25158414231165824. doi: 10.1177/25158414231165824. eCollection 2023 Jan-Dec. Ther Adv Ophthalmol. 2023. PMID: 37113304 Free PMC article.
-
Iron overload and iron chelating agent exposure in anemia-associated outer retinal degeneration: a case report and review of the literature.BMC Ophthalmol. 2021 Jul 13;21(1):277. doi: 10.1186/s12886-021-02030-1. BMC Ophthalmol. 2021. PMID: 34256738 Free PMC article. Review.
References
LinkOut - more resources
Full Text Sources
Other Literature Sources

