Precision Medicine for Paediatric Severe Asthma: Current Status and Future Direction
- PMID: 34045872
- PMCID: PMC8144021
- DOI: 10.2147/JAA.S265657
Precision Medicine for Paediatric Severe Asthma: Current Status and Future Direction
Erratum in
-
Erratum: Precision Medicine for Paediatric Severe Asthma: Current Status and Future Direction [Corrigendum].J Asthma Allergy. 2021 Jul 2;14:785-787. doi: 10.2147/JAA.S321964. eCollection 2021. J Asthma Allergy. 2021. PMID: 34239309 Free PMC article.
Abstract
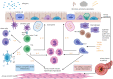
Asthma is a heterogeneous disease, characterised by different phenotypes and endotypes. Precision medicine in asthma refers to the implementation of a targeted therapy for each individual child, based on the identification of treatable traits, including environmental, immunological and genetic factors. Severe asthma in children is associated with increased hospitalisation rates, a lower quality of life, increased healthcare costs and an increased mortality. In the era of new molecular biologics treatments, it is essential to improve deep phenotyping of children with severe asthma in order to deliver the most effective treatment to each individual child. In this review, we discuss the personalised approach to the assessment and management of severe asthma. We explore the indications and use of the currently licensed biologics, as well as the potential of other emerging treatments.
Keywords: benralizumab; child; dupilumab; mepolizumab; omalizumab; reslizumab.
© 2021 Ramphul et al.
Conflict of interest statement
Dr Erol A Gaillard reports consultancy work for Boehringer Ingelheim with money paid to the institution (University of Leicester), investigator led research grants from Chiesi, Gilead,and Circassia, and non-financial support from Medimmune, outside the submitted work. The authors reported no other potential conflicts of interest for this work.
Figures

References
Publication types
LinkOut - more resources
Full Text Sources
Other Literature Sources

