Engineered Axonal Tracts as "Living Electrodes" for Synaptic-Based Modulation of Neural Circuitry
- PMID: 34045935
- PMCID: PMC8152180
- DOI: 10.1002/adfm.201701183
Engineered Axonal Tracts as "Living Electrodes" for Synaptic-Based Modulation of Neural Circuitry
Abstract
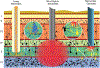
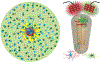
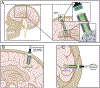
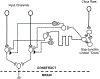
Brain-computer interface and neuromodulation strategies relying on penetrating non-organic electrodes/optrodes are limited by an inflammatory foreign body response that ultimately diminishes performance. A novel "biohybrid" strategy is advanced, whereby living neurons, biomaterials, and microelectrode/optical technology are used together to provide a biologically-based vehicle to probe and modulate nervous-system activity. Microtissue engineering techniques are employed to create axon-based "living electrodes", which are columnar microstructures comprised of neuronal population(s) projecting long axonal tracts within the lumen of a hydrogel designed to chaperone delivery into the brain. Upon microinjection, the axonal segment penetrates to prescribed depth for synaptic integration with local host neurons, with the perikaryal segment remaining externalized below conforming electrical-optical arrays. In this paradigm, only the biological component ultimately remains in the brain, potentially attenuating a chronic foreign-body response. Axon-based living electrodes are constructed using multiple neuronal subtypes, each with differential capacity to stimulate, inhibit, and/or modulate neural circuitry based on specificity uniquely afforded by synaptic integration, yet ultimately computer controlled by optical/electrical components on the brain surface. Current efforts are assessing the efficacy of this biohybrid interface for targeted, synaptic-based neuromodulation, and the specificity, spatial density and long-term fidelity versus conventional microelectronic or optical substrates alone.
Keywords: biologically-mediated neuromodulation; brain–computer interfaces; living scaffolds; microtissue engineering; tissue engineering.
Conflict of interest statement
Conflict of Interest The authors declare no conflict of interest.
Figures

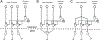
Similar articles
-
Bioactive Neuroelectronic Interfaces.Front Neurosci. 2019 Mar 29;13:269. doi: 10.3389/fnins.2019.00269. eCollection 2019. Front Neurosci. 2019. PMID: 30983957 Free PMC article. Review.
-
Development of optically controlled "living electrodes" with long-projecting axon tracts for a synaptic brain-machine interface.Sci Adv. 2021 Jan 22;7(4):eaay5347. doi: 10.1126/sciadv.aay5347. Print 2021 Jan. Sci Adv. 2021. PMID: 33523957 Free PMC article.
-
Anatomically Inspired Three-dimensional Micro-tissue Engineered Neural Networks for Nervous System Reconstruction, Modulation, and Modeling.J Vis Exp. 2017 May 31;(123):55609. doi: 10.3791/55609. J Vis Exp. 2017. PMID: 28605376 Free PMC article.
-
Restoring nervous system structure and function using tissue engineered living scaffolds.Neural Regen Res. 2015 May;10(5):679-85. doi: 10.4103/1673-5374.156943. Neural Regen Res. 2015. PMID: 26109930 Free PMC article.
-
Neural tissue engineering for neuroregeneration and biohybridized interface microsystems in vivo (Part 2).Crit Rev Biomed Eng. 2011;39(3):241-59. doi: 10.1615/critrevbiomedeng.v39.i3.40. Crit Rev Biomed Eng. 2011. PMID: 21967304 Review.
Cited by
-
Restoring lost nigrostriatal fibers in Parkinson's disease based on clinically-inspired design criteria.Brain Res Bull. 2021 Oct;175:168-185. doi: 10.1016/j.brainresbull.2021.07.016. Epub 2021 Jul 28. Brain Res Bull. 2021. PMID: 34332016 Free PMC article. Review.
-
Engineering strategies towards overcoming bleeding and glial scar formation around neural probes.Cell Tissue Res. 2022 Mar;387(3):461-477. doi: 10.1007/s00441-021-03567-9. Epub 2022 Jan 14. Cell Tissue Res. 2022. PMID: 35029757 Free PMC article. Review.
-
Angiogenic Cell Precursors and Neural Cell Precursors in Service to the Brain-Computer Interface.Cells. 2025 Jul 29;14(15):1163. doi: 10.3390/cells14151163. Cells. 2025. PMID: 40801596 Free PMC article. Review.
-
Neuromodulation of metabolic functions: from pharmaceuticals to bioelectronics to biocircuits.J Biol Eng. 2019 Aug 1;13:67. doi: 10.1186/s13036-019-0194-z. eCollection 2019. J Biol Eng. 2019. PMID: 31388355 Free PMC article. Review.
-
Gels, jets, mosquitoes, and magnets: a review of implantation strategies for soft neural probes.J Neural Eng. 2020 Sep 11;17(4):041002. doi: 10.1088/1741-2552/abacd7. J Neural Eng. 2020. PMID: 32759476 Free PMC article. Review.
References
-
- Duda JE, Struzyna LA, Browne KD, Chen HI, Wolf JA, Cullen DK, Proceedings of 21st International Congress of Parkinson’s Disease and Movement Disorders 2017.
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
