Reciprocal Relationship Between Calcium Signaling and Circadian Clocks: Implications for Calcium Homeostasis, Clock Function, and Therapeutics
- PMID: 34045944
- PMCID: PMC8144308
- DOI: 10.3389/fnmol.2021.666673
Reciprocal Relationship Between Calcium Signaling and Circadian Clocks: Implications for Calcium Homeostasis, Clock Function, and Therapeutics
Abstract
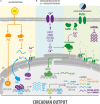
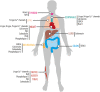
In animals, circadian clocks impose a daily rhythmicity to many behaviors and physiological processes. At the molecular level, circadian rhythms are driven by intracellular transcriptional/translational feedback loops (TTFL). Interestingly, emerging evidence indicates that they can also be modulated by multiple signaling pathways. Among these, Ca2+ signaling plays a key role in regulating the molecular rhythms of clock genes and of the resulting circadian behavior. In addition, the application of in vivo imaging approaches has revealed that Ca2+ is fundamental to the synchronization of the neuronal networks that make up circadian pacemakers. Conversely, the activity of circadian clocks may influence Ca2+ signaling. For instance, several genes that encode Ca2+ channels and Ca2+-binding proteins display a rhythmic expression, and a disruption of this cycling affects circadian function, underscoring their reciprocal relationship. Here, we review recent advances in our understanding of how Ca2+ signaling both modulates and is modulated by circadian clocks, focusing on the regulatory mechanisms described in Drosophila and mice. In particular, we examine findings related to the oscillations in intracellular Ca2+ levels in circadian pacemakers and how they are regulated by canonical clock genes, neuropeptides, and light stimuli. In addition, we discuss how Ca2+ rhythms and their associated signaling pathways modulate clock gene expression at the transcriptional and post-translational levels. We also review evidence based on transcriptomic analyzes that suggests that mammalian Ca2+ channels and transporters (e.g., ryanodine receptor, ip3r, serca, L- and T-type Ca2+ channels) as well as Ca2+-binding proteins (e.g., camk, cask, and calcineurin) show rhythmic expression in the central brain clock and in peripheral tissues such as the heart and skeletal muscles. Finally, we discuss how the discovery that Ca2+ signaling is regulated by the circadian clock could influence the efficacy of pharmacotherapy and the outcomes of clinical interventions.
Keywords: Drosophila; E-box; biological clocks; chronomedicine; circadian rhythms; daily rhythms.
Copyright © 2021 Cavieres-Lepe and Ewer.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures

Similar articles
-
Phase coupling of a circadian neuropeptide with rest/activity rhythms detected using a membrane-tethered spider toxin.PLoS Biol. 2008 Nov 4;6(11):e273. doi: 10.1371/journal.pbio.0060273. PLoS Biol. 2008. PMID: 18986214 Free PMC article.
-
Genetics and molecular biology of rhythms in Drosophila and other insects.Adv Genet. 2003;48:1-280. doi: 10.1016/s0065-2660(03)48000-0. Adv Genet. 2003. PMID: 12593455 Review.
-
Contribution of membrane-associated oscillators to biological timing at different timescales.Front Physiol. 2024 Jan 9;14:1243455. doi: 10.3389/fphys.2023.1243455. eCollection 2023. Front Physiol. 2024. PMID: 38264332 Free PMC article.
-
Post-transcriptional modulators and mediators of the circadian clock.Chronobiol Int. 2021 Sep;38(9):1244-1261. doi: 10.1080/07420528.2021.1928159. Epub 2021 May 31. Chronobiol Int. 2021. PMID: 34056966 Free PMC article. Review.
-
Circadian Rhythms and Sleep in Drosophila melanogaster.Genetics. 2017 Apr;205(4):1373-1397. doi: 10.1534/genetics.115.185157. Genetics. 2017. PMID: 28360128 Free PMC article. Review.
Cited by
-
Single-cell transcriptomics reveals that glial cells integrate homeostatic and circadian processes to drive sleep-wake cycles.Nat Neurosci. 2024 Feb;27(2):359-372. doi: 10.1038/s41593-023-01549-4. Epub 2024 Jan 23. Nat Neurosci. 2024. PMID: 38263460 Free PMC article.
-
The Mediation Effect of Peripheral Biomarkers of Calcium Metabolism and Chronotypes in Bipolar Disorder Psychopathology.Metabolites. 2022 Sep 2;12(9):827. doi: 10.3390/metabo12090827. Metabolites. 2022. PMID: 36144231 Free PMC article.
-
Pruritus and Neuropsychiatric Symptoms Among Patients with Darier Disease-An Overlooked and Interconnected Challenge.J Clin Med. 2025 Mar 8;14(6):1818. doi: 10.3390/jcm14061818. J Clin Med. 2025. PMID: 40142630 Free PMC article.
-
Functionally Validating Evolutionary Conserved Risk Genes for Parkinson's Disease in Drosophila melanogaster.Insects. 2023 Feb 9;14(2):168. doi: 10.3390/insects14020168. Insects. 2023. PMID: 36835737 Free PMC article.
-
In-phasic cytosolic-nuclear Ca2+ rhythms in suprachiasmatic nucleus neurons.Front Neurosci. 2023 Dec 20;17:1323565. doi: 10.3389/fnins.2023.1323565. eCollection 2023. Front Neurosci. 2023. PMID: 38178840 Free PMC article.
References
-
- Agostino P. V., Ferreyra G. A., Murad A. D., Watanabe Y., Golombek D. A. (2004). Diurnal, circadian and photic regulation of calcium/calmodulin-dependent kinase II and neuronal nitric oxide synthase in the hamster suprachiasmatic nuclei. Neurochem. Int. 44 617–625. 10.1016/j.neuint.2003.09.005 - DOI - PubMed
Publication types
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Miscellaneous

