Zerumbone Suppresses the LPS-Induced Inflammatory Response and Represses Activation of the NLRP3 Inflammasome in Macrophages
- PMID: 34045963
- PMCID: PMC8144706
- DOI: 10.3389/fphar.2021.652860
Zerumbone Suppresses the LPS-Induced Inflammatory Response and Represses Activation of the NLRP3 Inflammasome in Macrophages
Abstract
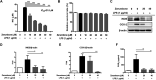
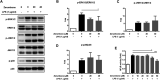
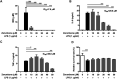
Zerumbone is a natural product isolated from the pinecone or shampoo ginger, Zingiber zerumbet (L.) Smith, which has a wide range of pharmacological activities, including anti-inflammatory effects. However, the effects of zerumbone on activation of the NLRP3 inflammasome in macrophages have not been examined. This study aimed to examine the effects of zerumbone on LPS-induced inflammatory responses and NLRP3 inflammasome activation using murine J774A.1 cells, murine peritoneal macrophages, and murine bone marrow-derived macrophages. Cells were treated with zerumbone following LPS or LPS/ATP treatment. Production of nitric oxide (NO) was measured by Griess reagent assay. The levels of IL-6, TNF-α, and IL-1β secretion were analyzed by ELISA. Western blotting analysis was performed to determine the expression of inducible NO synthase (iNOS), COX-2, MAPKs, and NLRP3 inflammasome-associated proteins. The activity of NF-κB was determined by a promoter reporter assay. The assembly of NLRP3 was examined by immunofluorescence staining and observed by confocal laser microscopy. Our experimental results indicated that zerumbone inhibited the production of NO, PGE2 and IL-6, suppressed the expression of iNOS and COX-2, repressed the phosphorylation of ERK, and decreased the activity of NF-κB in LPS-activated J774A.1 cells. In addition, zerumbone suppressed the production of IL-1β and inhibited the activity of NLRP3 inflammasome in LPS/ATP- and LPS/nigericin-activated J774A.1 cells. On the other hand, we also found that zerumbone repressed the production of NO and proinflammatory cytokines in LPS-activated murine peritoneal macrophages and bone marrow-derived macrophages. In conclusion, our experimental results demonstrate that zerumbone effectively attenuates the LPS-induced inflammatory response in macrophages both in vitro and ex vivo by suppressing the activation of the ERK-MAPK and NF-κB signaling pathways as well as blocking the activation of the NLRP3 inflammasome. These results imply that zerumbone may be beneficial for treating sepsis and inflammasome-related diseases.
Keywords: MAPKs; NLRP3 inflammasome; inflammation; macrophage; zerumbone.
Copyright © 2021 Su, Wang, Chen, Chiu, Liu, Huang, Huang, Fang, Cheng, Huang, Yeh, Liu, Lee, Huang and Li.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures
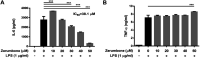




References
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

