Benchmarking the Extent and Speed of Reperfusion: First Pass TICI 2c-3 Is a Preferred Endovascular Reperfusion Endpoint
- PMID: 34046008
- PMCID: PMC8144635
- DOI: 10.3389/fneur.2021.669934
Benchmarking the Extent and Speed of Reperfusion: First Pass TICI 2c-3 Is a Preferred Endovascular Reperfusion Endpoint
Abstract
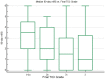
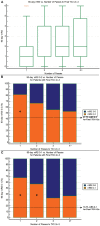
Background and Purpose: End-of-procedure substantial reperfusion [modified Treatment in Cerebral Ischemia (mTICI) 2b-3], the leading endpoint for thrombectomy studies, has several limitations including a ceiling effect, with recent achieved rates of ~90%. We aimed to identify a more optimal definition of angiographic success along two dimensions: (1) the extent of tissue reperfusion, and (2) the speed of revascularization. Methods: Core-lab adjudicated TICI scores for the first three passes of EmboTrap and the final all-procedures result were analyzed in the ARISE II multicenter study. The clinical impact of extent of reperfusion and speed of reperfusion (first-pass vs. later-pass) were evaluated. Clinical outcomes included 90-day functional independence [modified Rankin Scale (mRS) 0-2], 90-day freedom-from-disability (mRS 0-1), and dramatic early improvement [24-h National Institutes of Health Stroke Scale (NIHSS) improvement ≥ 8 points]. Results: Among 161 ARISE II subjects with ICA or MCA M1 occlusions, reperfusion results at procedure end showed substantial reperfusion in 149 (92.5%), excellent reperfusion in 121 (75.2%), and complete reperfusion in 79 (49.1%). Reperfusion rates on first pass were substantial in 81 (50.3%), excellent reperfusion in 62 (38.5%), and complete reperfusion in 44 (27.3%). First-pass excellent reperfusion (first-pass TICI 2c-3) had the greatest nominal predictive value for 90-day mRS 0-2 (sensitivity 58.5%, specificity 68.6%). There was a progressive worsening of outcomes with each additional pass required to achieve TICI 2c-3. Conclusions: First-pass excellent reperfusion (TICI 2c-3), reflecting rapid achievement of extensive reperfusion, is the technical revascularization endpoint that best predicted functional independence in this international multicenter trial and is an attractive candidate for a lead angiographic endpoint for future trials. Clinical Trial Registration: http://www.clinicaltrials.gov, identifier NCT02488915.
Keywords: brain ischaemia; cerebral infacrction; intra-arterial therapy; mechanical thrombectomy; reperfusion; reperfusion grading; stent retriever.
Copyright © 2021 Yoo, Soomro, Andersson, Saver, Ribo, Bozorgchami, Dabus, Liebeskind, Jadhav, Mattle and Zaidat.
Conflict of interest statement
AY is a consultant for Cerenovus, Penumbra, and Zoll, receives research grants from Medtronic, Cerenovus, Penumbra, Stryker, and Genentech, and has equity interest in Insera Therapeutics. TA is a consultant for Neuravi, Ablynx, Amnis Therapeutics, Medtronic, Rapid Medical, and Stryker. The University of California, Regents receives funding for JSa's services as a scientific consultant regarding trial design and conduct for Covidien and Stryker; JSa is an employee of the University of California, which holds a patent on retriever devices for stroke. MR is a shareholder in Anaconda Biomed, consultant for Cerenovus, Medtronic, Stryker, Apta Targets, and Vesalio. HB serves as a modest consultant for Cerenovus/Neuravi, and Stryker. GD serves as a consultant for Medtronic, Microvention, Penumbra, and Cerenovus. DL serves as an imaging core lab consultant for Cerenovus, Genentech, Medtronic, Stryker, and Vesalio. HM reports personal fees from Covidien/Medtronic, Neuravi/Cerenovus, Servier, and Bayer outside the submitted work, and served on the steering committees of the SWIFT PRIME and ARISE studies. OZ serves as a consultant for Neuravi, Stryker, Penumbra, and Medtronic. The remaining authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures

References
Associated data
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous

