Combining a Universal Telomerase Based Cancer Vaccine With Ipilimumab in Patients With Metastatic Melanoma - Five-Year Follow Up of a Phase I/IIa Trial
- PMID: 34046035
- PMCID: PMC8147687
- DOI: 10.3389/fimmu.2021.663865
Combining a Universal Telomerase Based Cancer Vaccine With Ipilimumab in Patients With Metastatic Melanoma - Five-Year Follow Up of a Phase I/IIa Trial
Abstract
Background: Ipilimumab improves survival for patients with metastatic malignant melanoma. Combining a therapeutic cancer vaccine with ipilimumab may increase efficacy by providing enhanced anti-tumor immune responses. UV1 consists of three synthetic long peptides from human telomerase reverse transcriptase (hTERT). These peptides comprise epitopes recognized by T cells from cancer patients experiencing long-term survival following treatment with a first-generation hTERT vaccine, and generate long-lasting immune responses in cancer patients when used as monotherapy. The objective of this trial was to investigate the safety and efficacy of combining UV1 with ipilimumab in metastatic melanoma.
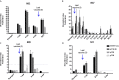
Patients and methods: In this phase I/IIa, single center trial [NCT02275416], patients with metastatic melanoma received repeated UV1 vaccinations, with GM-CSF as an adjuvant, in combination with ipilimumab. Patients were evaluated for safety, efficacy and immune response. Immune responses against vaccine peptides were monitored in peripheral blood by measuring antigen-specific proliferation and IFN-γ production.
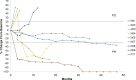
Results: Twelve patients were recruited. Adverse events were mainly diarrhea, injection site reaction, pruritus, rash, nausea and fatigue. Ten patients showed a Th1 immune response to UV1 peptides, occurring early and after few vaccinations. Three patients obtained a partial response and one patient a complete response. Overall survival was 50% at 5 years.
Conclusion: Treatment was well tolerated. The rapid expansion of UV1-specific Th1 cells in the majority of patients indicates synergy between UV1 vaccine and CTLA-4 blockade. This may have translated into clinical benefit, encouraging the combination of UV1 vaccination with standard of care treatment regimes containing ipilimumab/CTLA-4 blocking antibodies.
Keywords: ipilimumab; melanoma; peptide vaccine immunotherapy; phase I/IIa studies; telomerase (hTERT).
Copyright © 2021 Aamdal, Inderberg, Ellingsen, Rasch, Brunsvig, Aamdal, Heintz, Vodák, Nakken, Hovig, Nyakas, Guren and Gaudernack.
Conflict of interest statement
EMI and GG are inventors of a UV1 vaccine patent. EI, WR, GG and SA are shareholders in Ultimovacs ASA. EE, WR, SA and GG are employees of Ultimovacs ASA. MN has received personal honoraria from BMS for lectures. The authors declare that this study received funding from Ultimovacs ASA. The funder had the following involvement with the study: study design of the trial.
Figures


References
-
- Wei SC, Duffy CR, Allison JP. Fundamental Mechanisms of Immune Checkpoint Blockade Therapy Vol. 8. Cancer Discov (2018) 8(9):1069–86. 10.1158/2159-8290.CD-18-0367 - DOI - PubMed
Publication types
MeSH terms
Substances
Associated data
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases

