Effects of NSAIDs on pre-osteoblast viability and osteogenic differentiation
- PMID: 34046094
- PMCID: PMC8141960
- DOI: 10.3892/etm.2021.10172
Effects of NSAIDs on pre-osteoblast viability and osteogenic differentiation
Abstract
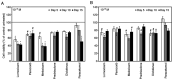
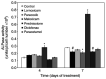
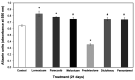
Non-steroidal anti-inflammatory drugs (NSAIDs) are widely used in the treatment of a variety of musculoskeletal conditions, injuries and after surgery for postoperative pain management. Their use has been associated with impaired bone healing, possibly due to a multifactorial function, which may include inhibition of osteoblast recruitment and differentiation. However, up to date, there is no consensus regarding the impact of NSAIDs on bone-healing. The aim of the current study was to investigate the effects of five NSAIDs on the cellular functions of mouse MC3T3-E1 pre-osteoblasts. Cells were treated with the non-selective COX inhibitors lornoxicam and diclofenac, the COX-2 selective inhibitors parecoxib, meloxicam and paracetamol, as well as steroidal prednisolone at different doses and exposure times. The PrestoBlue™ technique was used to measure cell viability, an enzymatic assay was employed for alkaline phosphatase (ALP) activity and alizarin red S mineral staining was used to determine osteogenic differentiation. All drugs had a negative impact on pre-osteoblast cell growth, with the exception of paracetamol. Lornoxicam, diclofenac and meloxicam reduced ALP activity, while the other NSAIDs had no effect and prednisolone strongly increased ALP activity. In contrast, calcium deposits were either unaffected or increased by NSAID treatments but were significantly decreased by prednisolone. These results provide evidence that NSAIDs may adversely affect the viability of mouse pre-osteoblast cells but their actions on the osteogenic differentiation are drug-specific. The direct comparison of the effects of different NSAIDs and prednisolone on pre-osteoblasts may serve to place some NSAIDs in a preferential position for analgesic and anti-inflammatory therapy during bone repair.
Keywords: MC3T3-E1; cell viability; non-steroidal anti-inflammatory drugs; osteogenic differentiation; prednisolone.
Copyright © 2020, Spandidos Publications.
Conflict of interest statement
The authors declare that they have no competing interests.
Figures

References
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
