Mechanisms of SARS-CoV-2-induced lung vascular disease: potential role of complement
- PMID: 34046161
- PMCID: PMC8138299
- DOI: 10.1177/20458940211015799
Mechanisms of SARS-CoV-2-induced lung vascular disease: potential role of complement
Abstract
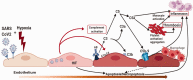
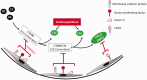
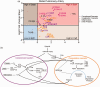
The outbreak of COVID-19 disease, caused by SARS-CoV-2 beta-coronovirus, urges a focused search for the underlying mechanisms and treatment options. The lung is the major target organ of COVID-19, wherein the primary cause of mortality is hypoxic respiratory failure, resulting from acute respiratory distress syndrome, with severe hypoxemia, often requiring assisted ventilation. While similar in some ways to acute respiratory distress syndrome secondary to other causes, lungs of some patients dying with COVID-19 exhibit distinct features of vascular involvement, including severe endothelial injury and cell death via apoptosis and/or pyroptosis, widespread capillary inflammation, and thrombosis. Furthermore, the pulmonary pathology of COVID-19 is characterized by focal inflammatory cell infiltration, impeding alveolar gas exchange resulting in areas of local tissue hypoxia, consistent with potential amplification of COVID-19 pathogenicity by hypoxia. Vascular endothelial cells play essential roles in both innate and adaptive immune responses, and are considered to be "conditional innate immune cells" centrally participating in various inflammatory, immune pathologies. Activated endothelial cells produce cytokines/chemokines, dynamically recruit and activate inflammatory cells and platelets, and centrally participate in pro-thrombotic processes (thrombotic microangiopathies). Initial reports presented pathological findings of localized direct infection of vascular endothelial cells with SARS-CoV-2, yet emerging evidence does not support direct infection of endothelial or other vascular wall cell and thus widespread endothelial cell dysfunction and inflammation may be better explained as secondary responses to epithelial cell infection and inflammation. Endothelial cells are also actively engaged in a cross-talk with the complement system, the essential arm of innate immunity. Recent reports present evidence for complement deposition in SARS-CoV-2-damaged lung microcirculation, further strengthening the idea that, in severe cases of COVID-19, complement activation is an essential player, generating destructive hemorrhagic, capillaritis-like tissue damage, clotting, and hyperinflammation. Thus, complement-targeted therapies are actively in development. This review is intended to explore in detail these ideas.
Keywords: COVID 19; complement; endothelial cell; hypoxia; inflammation.
© The Author(s) 2021.
Figures





References
Publication types
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

