The Crosstalk Between Cancer Cells and Neutrophils Enhances Hepatocellular Carcinoma Metastasis via Neutrophil Extracellular Traps-Associated Cathepsin G Component: A Potential Therapeutic Target
- PMID: 34046369
- PMCID: PMC8144903
- DOI: 10.2147/JHC.S303588
The Crosstalk Between Cancer Cells and Neutrophils Enhances Hepatocellular Carcinoma Metastasis via Neutrophil Extracellular Traps-Associated Cathepsin G Component: A Potential Therapeutic Target
Abstract
Background: Emerging evidences have highlighted the roles of neutrophils, as the major host microenvironment component, in the development of hepatocellular carcinoma (HCC). Neutrophils extracellular traps (NETs) produced in the infection can strengthen the behavior of cancer metastasis. Here, we investigated the roles of NETs in HCC metastasis and further explore the underlying mechanism of how NETs interact with cancer.
Methods: The neutrophils were isolated from whole blood of HCC patients and used to evaluate the formation of NETs. NET markers were detected in tissue samples, plasma and cell climbing slice. Mouse models were used to evaluate the roles of NETs in HCC metastasis in vivo, and the corresponding mechanisms were explored using in vivo and in vitro assays.
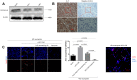
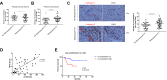
Results: An increase in the release of NETs in patients with HCC, particularly those with portal vein tumor thrombosis (PVTT). The presence of NETs in HCC tumor tissues closely correlated with a poor prognosis. Functionally, the invasion ability of HCC cells was enhanced by co-culture with HCC neutrophils, through NETs formation, while the neutrophils from a healthy donor (HD) exhibited the inhibition of the invasion ability. Furthermore, we observed an enhanced ability of forming NETs in neutrophils from HCC patients in vitro, especially patients with PVTT or extra-hepatic metastasis. An in-vivo animal study demonstrated that neutrophils of HCC facilitated the metastatic behavior towards the lung. The further mechanistic investigation unveiled that HCC cells-derived cytokine IL-8 triggered NETs formation in an NADPH oxidase-dependent manner, and NETs-associated cathepsin G (cG) promoted HCC metastasis in vitro as well as vivo. Clinically, the expression of the cG protein in tumor tissues displayed a close correlation with the disease prognosis of HCC patients.
Conclusion: Our findings implicated that the induction of NETs by HCC cells is a critical metastasis-supporting cancer-host interaction and that NETs may serve as an immune-based potential therapeutic target against HCC progression.
Keywords: E-cadherin; IL-8; NADPH; cathepsin G; hepatocellular carcinoma; metastasis; neutrophils extracellular traps.
© 2021 Guan et al.
Conflict of interest statement
The authors reported no conflicts of interest for this work.
Figures







Similar articles
-
Increased neutrophil extracellular traps promote metastasis potential of hepatocellular carcinoma via provoking tumorous inflammatory response.J Hematol Oncol. 2020 Jan 6;13(1):3. doi: 10.1186/s13045-019-0836-0. J Hematol Oncol. 2020. PMID: 31907001 Free PMC article.
-
Neutrophil extracellular traps induce tumor metastasis through dual effects on cancer and endothelial cells.Oncoimmunology. 2022 Mar 16;11(1):2052418. doi: 10.1080/2162402X.2022.2052418. eCollection 2022. Oncoimmunology. 2022. PMID: 35309732 Free PMC article.
-
Targeting SPP1-orchestrated neutrophil extracellular traps-dominant pre-metastatic niche reduced HCC lung metastasis.Exp Hematol Oncol. 2024 Nov 11;13(1):111. doi: 10.1186/s40164-024-00571-x. Exp Hematol Oncol. 2024. PMID: 39529085 Free PMC article.
-
The Emerging Role of Neutrophil Extracellular Traps (NETs) in Tumor Progression and Metastasis.Front Immunol. 2020 Sep 16;11:1749. doi: 10.3389/fimmu.2020.01749. eCollection 2020. Front Immunol. 2020. PMID: 33042107 Free PMC article. Review.
-
Neutrophil extracellular traps regulating tumorimmunity in hepatocellular carcinoma.Front Immunol. 2023 Dec 18;14:1253964. doi: 10.3389/fimmu.2023.1253964. eCollection 2023. Front Immunol. 2023. PMID: 38173719 Free PMC article. Review.
Cited by
-
Neutrophil extracellular traps in tumor progression and immunotherapy.Front Immunol. 2023 Mar 13;14:1135086. doi: 10.3389/fimmu.2023.1135086. eCollection 2023. Front Immunol. 2023. PMID: 36993957 Free PMC article. Review.
-
Lenvatinib-activated NDUFA4L2/IL33/PADI4 pathway induces neutrophil extracellular traps that inhibit cuproptosis in hepatocellular carcinoma.Cell Oncol (Dordr). 2025 Apr;48(2):487-504. doi: 10.1007/s13402-024-01013-w. Epub 2024 Nov 25. Cell Oncol (Dordr). 2025. PMID: 39585643 Free PMC article.
-
Neutrophil Extracellular Traps in Digestive Cancers: Warrior or Accomplice.Front Oncol. 2021 Nov 19;11:766636. doi: 10.3389/fonc.2021.766636. eCollection 2021. Front Oncol. 2021. PMID: 34868992 Free PMC article. Review.
-
The causal relationship between cathepsins and digestive system tumors: a Mendelian randomization study.Front Oncol. 2024 Mar 25;14:1365138. doi: 10.3389/fonc.2024.1365138. eCollection 2024. Front Oncol. 2024. PMID: 38590662 Free PMC article.
-
The multifaceted roles of cathepsins in immune and inflammatory responses: implications for cancer therapy, autoimmune diseases, and infectious diseases.Biomark Res. 2024 Dec 31;12(1):165. doi: 10.1186/s40364-024-00711-9. Biomark Res. 2024. PMID: 39736788 Free PMC article. Review.
References
-
- Papayannopoulos V. Neutrophil extracellular traps in immunity and disease. Nat Rev Immunol. 2018;18(2):134–147. - PubMed
LinkOut - more resources
Full Text Sources
Other Literature Sources

