Vitamin C Inhibits Blood-Stage Plasmodium Parasites via Oxidative Stress
- PMID: 34046404
- PMCID: PMC8144511
- DOI: 10.3389/fcell.2021.639944
Vitamin C Inhibits Blood-Stage Plasmodium Parasites via Oxidative Stress
Abstract
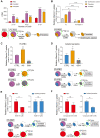
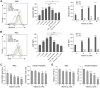
During the Plasmodium erythrocytic cycle, glucose is taken up by glucose transporters (GLUTs) in red blood cells (RBCs) and supplied to parasites via the Plasmodium hexose transporter. Here, we demonstrate that the glucose uptake pathway in infected RBCs (iRBCs) can be hijacked by vitamin C (Vc). GLUTs preferentially transport the oxidized form of Vc, which is subsequently reduced in the cytosol. Vc, which is expected to burden the intracellular reducing capacity, inhibits Plasmodium berghei and Plasmodium falciparum growth. Vc uptake is drastically increased in iRBCs, with a large proportion entering parasites. Increased absorption of Vc causes accumulation of reactive oxygen species, reduced ATP production, and elevated eryptosis in iRBCs and apoptosis in parasites. The level of oxidative stress induced by Vc is significantly higher in iRBCs than uninfected RBCs, not seen in chloroquine or artemisinin-treated iRBCs, and effective in inhibiting chloroquine or artemisinin-resistant parasites. These findings provide important insights into the drug sensitivity of Plasmodium.
Keywords: Plasmodium; eryptosis; glucose transporter; oxidative stress; vitamin C (ascorbic acid).
Copyright © 2021 Shi, Wei, Xu, Liu, Zhang, Lv and Wang.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures




Similar articles
-
Inhibition of GLUTs by WZB117 mediates apoptosis in blood-stage Plasmodium parasites by breaking redox balance.Biochem Biophys Res Commun. 2018 Sep 5;503(2):1154-1159. doi: 10.1016/j.bbrc.2018.06.134. Epub 2018 Aug 2. Biochem Biophys Res Commun. 2018. PMID: 29953861
-
Calcium transport and compartment analysis of free and exchangeable calcium in Plasmodium falciparum-infected red blood cells.J Protozool. 1991 Nov-Dec;38(6):594-601. J Protozool. 1991. PMID: 1667934
-
Deformability limits of Plasmodium falciparum-infected red blood cells.Cell Microbiol. 2009 Sep;11(9):1340-53. doi: 10.1111/j.1462-5822.2009.01334.x. Epub 2009 Apr 30. Cell Microbiol. 2009. PMID: 19438513 Free PMC article.
-
Why is the Plasmodium falciparum hexose transporter a promising new drug target?Expert Opin Ther Targets. 2003 Oct;7(5):593-602. doi: 10.1517/14728222.7.5.593. Expert Opin Ther Targets. 2003. PMID: 14498822 Review.
-
Manipulating Eryptosis of Human Red Blood Cells: A Novel Antimalarial Strategy?Front Cell Infect Microbiol. 2018 Nov 30;8:419. doi: 10.3389/fcimb.2018.00419. eCollection 2018. Front Cell Infect Microbiol. 2018. PMID: 30560094 Free PMC article. Review.
Cited by
-
Eryptosis: Programmed Death of Nucleus-Free, Iron-Filled Blood Cells.Cells. 2022 Feb 1;11(3):503. doi: 10.3390/cells11030503. Cells. 2022. PMID: 35159312 Free PMC article. Review.
-
Ascorbic Acid Ameriolates Liver Damage by Myeloperoxidase Oxidative Products in a Hamster Model of Amoebic Liver Abscess.Front Cell Infect Microbiol. 2022 Mar 22;12:855822. doi: 10.3389/fcimb.2022.855822. eCollection 2022. Front Cell Infect Microbiol. 2022. PMID: 35392606 Free PMC article.
-
Dobinin K Displays Antiplasmodial Activity through Disruption of Plasmodium falciparum Mitochondria and Generation of Reactive Oxygen Species.Molecules. 2024 Oct 8;29(19):4759. doi: 10.3390/molecules29194759. Molecules. 2024. PMID: 39407688 Free PMC article.
-
Mannose inhibits Plasmodium parasite growth and cerebral malaria development via regulation of host immune responses.Front Immunol. 2022 Sep 23;13:859228. doi: 10.3389/fimmu.2022.859228. eCollection 2022. Front Immunol. 2022. PMID: 36211381 Free PMC article.
-
Therapeutic potential role of vitamin C in prevention and control of heart transplant rejection and cardiac allograft vasculopathy. A need for consideration.Health Sci Rep. 2023 Nov 5;6(11):e1687. doi: 10.1002/hsr2.1687. eCollection 2023 Nov. Health Sci Rep. 2023. PMID: 37936616 Free PMC article.
References
LinkOut - more resources
Full Text Sources
Other Literature Sources

