Ivacaftor Inhibits Glioblastoma Stem Cell Maintenance and Tumor Progression
- PMID: 34046412
- PMCID: PMC8147559
- DOI: 10.3389/fcell.2021.678209
Ivacaftor Inhibits Glioblastoma Stem Cell Maintenance and Tumor Progression
Abstract
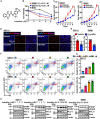
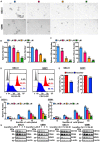
Glioblastoma (GBM) is the most common and malignant primary brain tumor. Glioblastoma stem cells (GSCs) not only initiate and sustain uncontrolled cell proliferation but also resistant to conventional clinical therapies including temozolomide (TMZ) dependent chemotherapy and radiotherapy, implying that there is an urgent need to identify new therapeutic strategies especially specific targeting GSCs. Here, we provide evidence showing that ivacaftor commonly applied in cystic fibrosis therapy acts as a potent inhibitor for GSCs maintenance. We found that ivacaftor promotes cellular apoptosis in vitro and represses patient-derived xenograft (PDX) tumor growth in vivo. In addition, we demonstrate that ivacaftor decreases stemness marker gene expressions of GSCs, including CD133, CD44, and Sox2. In summary, our findings reveal that ivacaftor inhibits glioblastoma progression via specifically eliminating GSCs, which opens a new avenue for GBM clinical therapy in the future.
Keywords: apoptosis; glioblastoma; glioblastoma stem cell; ivacaftor; stemness.
Copyright © 2021 Liu, Pu, Nie, Shi, Jiang, Wu, Chen and Yang.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures


Similar articles
-
Dual-targeting immunoliposomes using angiopep-2 and CD133 antibody for glioblastoma stem cells.J Control Release. 2018 Jan 10;269:245-257. doi: 10.1016/j.jconrel.2017.11.026. Epub 2017 Nov 21. J Control Release. 2018. PMID: 29162480
-
Morusin inhibits glioblastoma stem cell growth in vitro and in vivo through stemness attenuation, adipocyte transdifferentiation, and apoptosis induction.Mol Carcinog. 2016 Jan;55(1):77-89. doi: 10.1002/mc.22260. Epub 2014 Dec 31. Mol Carcinog. 2016. PMID: 25557841
-
Engagement of cellular prion protein with the co-chaperone Hsp70/90 organizing protein regulates the proliferation of glioblastoma stem-like cells.Stem Cell Res Ther. 2017 Apr 17;8(1):76. doi: 10.1186/s13287-017-0518-1. Stem Cell Res Ther. 2017. PMID: 28412969 Free PMC article.
-
Targeting Glioblastoma Stem Cells to Overcome Chemoresistance: An Overview of Current Therapeutic Strategies.Biomedicines. 2022 Jun 2;10(6):1308. doi: 10.3390/biomedicines10061308. Biomedicines. 2022. PMID: 35740330 Free PMC article. Review.
-
Mitochondria's Role in the Maintenance of Cancer Stem Cells in Glioblastoma.Front Oncol. 2021 Feb 22;11:582694. doi: 10.3389/fonc.2021.582694. eCollection 2021. Front Oncol. 2021. PMID: 33692947 Free PMC article. Review.
Cited by
-
Tumor-suppressive function and mechanism of miR-873-5p in glioblastoma: evidence based on bioinformatics analysis and experimental validation.Aging (Albany NY). 2023 Jun 28;15(12):5412-5425. doi: 10.18632/aging.204800. Epub 2023 Jun 28. Aging (Albany NY). 2023. PMID: 37382594 Free PMC article.
-
Cell-based and cell-free immunotherapies for glioblastoma: current status and future directions.Front Immunol. 2023 May 25;14:1175118. doi: 10.3389/fimmu.2023.1175118. eCollection 2023. Front Immunol. 2023. PMID: 37304305 Free PMC article. Review.
-
Inhibition of STAT3 signaling as critical molecular event in HUC-MSCs suppressed Glioblastoma Cells.J Cancer. 2023 Feb 27;14(4):611-627. doi: 10.7150/jca.77905. eCollection 2023. J Cancer. 2023. PMID: 37057281 Free PMC article.
-
Long noncoding RNA ADIRF antisense RNA 1 upregulates insulin receptor substrate 1 to decrease the aggressiveness of osteosarcoma by sponging microRNA-761.Bioengineered. 2022 Feb;13(2):2028-2043. doi: 10.1080/21655979.2021.2019872. Bioengineered. 2022. PMID: 35030964 Free PMC article.
-
The Emerging Landscapes of Long Noncoding RNA in Thyroid Carcinoma: Biological Functions and Clinical Significance.Front Oncol. 2021 Aug 10;11:706011. doi: 10.3389/fonc.2021.706011. eCollection 2021. Front Oncol. 2021. PMID: 34447696 Free PMC article. Review.
References
-
- Das S., Srikanth M., Kessler J. A. (2008). Cancer stem cells and glioma. Nat. Clin. Pract. Neurol. 4 427–435. - PubMed
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

