Synthesis and σ receptor affinity of spiro[[2]benzopyran-1,1'-cyclohexanes] with an exocyclic amino moiety in the 3'-position
- PMID: 34046612
- PMCID: PMC8127328
- DOI: 10.1039/d0md00307g
Synthesis and σ receptor affinity of spiro[[2]benzopyran-1,1'-cyclohexanes] with an exocyclic amino moiety in the 3'-position
Abstract
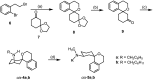
The main functions of σ 1 receptors include the modulation of release and reuptake of neurotransmitters, the regulation of ion channels and the influence on intracellular signaling through modulation of calcium levels. Due to these properties, σ 1 receptors are interesting drug targets for the treatment of various neurological disorders, pain and cancer. In order to modify the distance between the pharmacophoric elements (the benzene ring of 2-benzopyran and an amino moiety), a set of spiro[[2]benzopyran-1,1'-cyclohexan]-3'-amines was synthesized. The key step of the synthesis was a Parham cyclization of 1-bromo-2-(2-bromoethyl)benzene (6) with the mono ketal 7 of cyclohexane-1,3-dione, which led in a one-pot reaction to the spirocyclic framework 8. Reductive amination of ketone 9 stereoselectively provided secondary amines cis-4, which were methylated to afford tertiary amines cis-5. Whereas spirocyclic compounds cis-4a and cis-5a bearing a benzyl moiety at the exocyclic amino moiety showed rather low σ 1 affinity, the corresponding cyclohexylmethyl derivatives cis-4b and cis-5b exhibited low nanomolar σ 1 affinity. The secondary amine cis-4b displayed the highest σ 1 receptor affinity (K i = 5.4 nM) in this series. Methylation of the secondary amine cis-4b led to a slightly decreased σ 1 receptor affinity of cis-5b (K i = 15 nM).
This journal is © The Royal Society of Chemistry.
Conflict of interest statement
The authors declare no conflict of interest.
Figures

Similar articles
-
Structure-Affinity Relationships of Fluorinated Spirocyclic σ2 Receptor Ligands with an Exocyclic Benzylamino Moiety.ChemMedChem. 2019 Aug 6;14(15):1392-1402. doi: 10.1002/cmdc.201900284. Epub 2019 Jul 12. ChemMedChem. 2019. PMID: 31189022
-
Synthesis and Structure-Affinity Relationships of Spirocyclic Benzopyrans with Exocyclic Amino Moiety.J Med Chem. 2019 Apr 25;62(8):4204-4217. doi: 10.1021/acs.jmedchem.9b00449. Epub 2019 Apr 12. J Med Chem. 2019. PMID: 30939014
-
Synthesis of σ Receptor Ligands with a Spirocyclic System Connected with a Tetrahydroisoquinoline Moiety via Different Linkers.ChemMedChem. 2021 Apr 8;16(7):1184-1197. doi: 10.1002/cmdc.202000861. Epub 2021 Feb 2. ChemMedChem. 2021. PMID: 33332704 Free PMC article.
-
Design, synthesis and pharmacological evaluation of spirocyclic σ(1) receptor ligands with exocyclic amino moiety (increased distance 1).Bioorg Med Chem. 2011 May 15;19(10):3141-51. doi: 10.1016/j.bmc.2011.04.002. Epub 2011 Apr 6. Bioorg Med Chem. 2011. PMID: 21531141
-
Pharmacophore models and development of spirocyclic ligands for σ1 receptors.Curr Pharm Des. 2012;18(7):930-7. doi: 10.2174/138161212799436548. Curr Pharm Des. 2012. PMID: 22288413 Review.
References
-
- Martin W. R. Eades C. G. Thomson J. A. Huppler R. E. Gilbert P. E. The effects of morphine- and nalorphine- like drugs in the nondependent and morphine-dependent chronic spinal dog. J. Pharmacol. Exp. Ther. 1976;197:517–532. - PubMed
LinkOut - more resources
Full Text Sources
Other Literature Sources
Chemical Information

