Drugging the undruggable: a computational chemist's view of KRASG12C
- PMID: 34046632
- PMCID: PMC8128063
- DOI: 10.1039/d1md00055a
Drugging the undruggable: a computational chemist's view of KRASG12C
Abstract
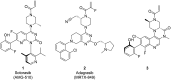
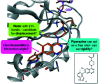
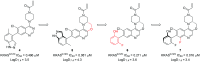
In recent years, the emergence of targeted covalent inhibitors which bind to the G12C mutant of KRAS have offered a solution to this previously intractable target. Inhibitors of KRASG12C tend to be structurally complex, displaying features such as atropisomerism, chiral centres and a reactive covalent warhead. Such molecules result in lengthy and challenging syntheses, and as a consequence critical decisions need to be made at the design level to maximise the chances of success. Here we take a retrospective look into how computational chemistry can help guide and prioritise medicinal chemistry efforts in the context of a series of conformationally restricted tetracyclic quinolines.
This journal is © The Royal Society of Chemistry.
Conflict of interest statement
There are no conflicts to declare.
Figures
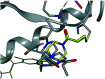
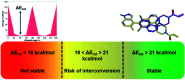
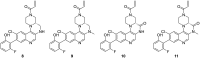


References
-
- Hillig R. C. Sautier B. Schroeder J. Moosmayer D. Hilpmann A. Stegmann C. M. Werbeck N. D. Briem H. Boemer U. Weiske J. Badock V. Mastouri J. Petersen K. Siemeister G. Kahmann J. D. Wegener D. Böhnke N. Eis K. Graham K. Wortmann L. Von Nussbaum F. Bader B. Proc. Natl. Acad. Sci. U. S. A. 2019;116:2551–2560. - PMC - PubMed
-
- Winter J. J. G. Anderson M. Blades K. Brassington C. Breeze A. L. Chresta C. Embrey K. Fairley G. Faulder P. Finlay M. R. V. Kettle J. G. Nowak T. Overman R. Patel S. J. Perkins P. Spadola L. Tart J. Tucker J. A. Wrigley G. J. Med. Chem. 2015;58:2265–2274. - PubMed
LinkOut - more resources
Full Text Sources
Other Literature Sources
Chemical Information
Miscellaneous

