Neural crest multipotency and specification: power and limits of single cell transcriptomic approaches
- PMID: 34046642
- PMCID: PMC8130411
- DOI: 10.12703/r/10-38
Neural crest multipotency and specification: power and limits of single cell transcriptomic approaches
Abstract
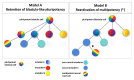
The neural crest is a unique population of multipotent cells forming in vertebrate embryos. Their vast cell fate potential enables the generation of a diverse array of differentiated cell types in vivo. These include, among others, connective tissue, cartilage and bone of the face and skull, neurons and glia of the peripheral nervous system (including enteric nervous system), and melanocytes. Following migration, these derivatives extensively populate multiple germ layers. Within the competent neural border ectoderm, an area located at the junction between the neural and non-neural ectoderm during embryonic development, neural crest cells form in response to a series of inductive secreted cues including BMP, Wnt, and FGF signals. As cells become progressively specified, they express transcriptional modules conducive with their stage of fate determination or cell state. Those sequential states include the neural border state, the premigratory neural crest state, the epithelium-to-mesenchyme transitional state, and the migratory state to end with post-migratory and differentiation states. However, despite the extensive knowledge accumulated over 150 years of neural crest biology, many key questions remain open, in particular the timing of neural crest lineage determination, the control of potency during early developmental stages, and the lineage relationships between different subpopulations of neural crest cells. In this review, we discuss the recent advances in understanding early neural crest formation using cutting-edge high-throughput single cell sequencing approaches. We will discuss how this new transcriptomic data, from 2017 to 2021, has advanced our knowledge of the steps in neural crest cell lineage commitment and specification, the mechanisms driving multipotency, and diversification. We will then discuss the questions that remain to be resolved and how these approaches may continue to unveil the biology of these fascinating cells.
Keywords: fate specification; lineage specification; multipotency; neural crest; pluripotency; single cell transcriptomics.
Copyright: © 2021 Artinger KB et al.
Conflict of interest statement
The authors declare that they have no competing interests.No competing interests were disclosed.No competing interests were disclosed.No competing interests were disclosed.
Figures

Similar articles
-
[Phenotypic plasticity of neural crest-derived melanocytes and Schwann cells].Biol Aujourdhui. 2011;205(1):53-61. doi: 10.1051/jbio/2011008. Epub 2011 Apr 19. Biol Aujourdhui. 2011. PMID: 21501576 Review. French.
-
Transcriptional control of neural crest specification into peripheral glia.Glia. 2015 Nov;63(11):1883-1896. doi: 10.1002/glia.22816. Epub 2015 Mar 10. Glia. 2015. PMID: 25752517 Review.
-
Specification and formation of the neural crest: Perspectives on lineage segregation.Genesis. 2019 Jan;57(1):e23276. doi: 10.1002/dvg.23276. Epub 2019 Jan 15. Genesis. 2019. PMID: 30576078 Free PMC article. Review.
-
Seq Your Destiny: Neural Crest Fate Determination in the Genomic Era.Annu Rev Genet. 2021 Nov 23;55:349-376. doi: 10.1146/annurev-genet-071719-020954. Epub 2021 Sep 21. Annu Rev Genet. 2021. PMID: 34546797
-
Molecular mechanisms of neural crest formation.Annu Rev Cell Dev Biol. 1999;15:81-112. doi: 10.1146/annurev.cellbio.15.1.81. Annu Rev Cell Dev Biol. 1999. PMID: 10611958 Review.
Cited by
-
Melanocyte lineage dynamics in development, growth and disease.Development. 2024 Aug 1;151(15):dev201266. doi: 10.1242/dev.201266. Epub 2024 Aug 2. Development. 2024. PMID: 39092608 Free PMC article. Review.
-
Bridging the gap between omics research and dental practice.BDJ Open. 2024 Mar 4;10(1):16. doi: 10.1038/s41405-024-00199-3. BDJ Open. 2024. PMID: 38438363 Free PMC article. Review.
-
Spatiotemporal single-cell regulatory atlas reveals neural crest lineage diversification and cellular function during tooth morphogenesis.Nat Commun. 2022 Aug 16;13(1):4803. doi: 10.1038/s41467-022-32490-y. Nat Commun. 2022. PMID: 35974052 Free PMC article.
-
Time to go: neural crest cell epithelial-to-mesenchymal transition.Development. 2022 Aug 1;149(15):dev200712. doi: 10.1242/dev.200712. Epub 2022 Jul 29. Development. 2022. PMID: 35905012 Free PMC article. Review.
-
NRN1 interacts with Notch to increase oncogenic STAT3 signaling in melanoma.Cell Commun Signal. 2024 May 6;22(1):256. doi: 10.1186/s12964-024-01632-8. Cell Commun Signal. 2024. PMID: 38705997 Free PMC article.
References
Publication types
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
