Pericyte hypoxia-inducible factor-1 (HIF-1) drives blood-brain barrier disruption and impacts acute ischemic stroke outcome
- PMID: 34046769
- PMCID: PMC8487886
- DOI: 10.1007/s10456-021-09796-4
Pericyte hypoxia-inducible factor-1 (HIF-1) drives blood-brain barrier disruption and impacts acute ischemic stroke outcome
Abstract
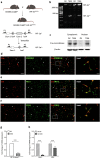
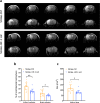
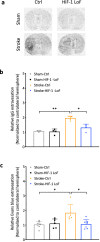
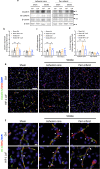
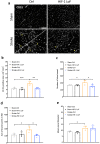
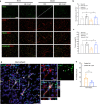
Pericytes play essential roles in blood-brain barrier integrity and their dysfunction is implicated in neurological disorders such as stroke although the underlying mechanisms remain unknown. Hypoxia-inducible factor-1 (HIF-1), a master regulator of injury responses, has divergent roles in different cells especially during stress scenarios. On one hand HIF-1 is neuroprotective but on the other it induces vascular permeability. Since pericytes are critical for barrier stability, we asked if pericyte HIF-1 signaling impacts barrier integrity and injury severity in a mouse model of ischemic stroke. We show that pericyte HIF-1 loss of function (LoF) diminishes ischemic damage and barrier permeability at 3 days reperfusion. HIF-1 deficiency preserved barrier integrity by reducing pericyte death thereby maintaining vessel coverage and junctional protein organization, and suppressing vascular remodeling. Importantly, considerable improvements in sensorimotor function were observed in HIF-1 LoF mice indicating that better vascular functionality post stroke improves outcome. Thus, boosting vascular integrity by inhibiting pericytic HIF-1 activation and/or increasing pericyte survival may be a lucrative option to accelerate recovery after severe brain injury.
Keywords: Cerebral ischemia; Pericyte coverage; Pericyte death; Vascular permeability.
© 2021. The Author(s).
Conflict of interest statement
The authors declare that they have no conflict of interest.
Figures


References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

