Slounase, a Batroxobin Containing Activated Factor X Effectively Enhances Hemostatic Clot Formation and Reducing Bleeding in Hypocoagulant Conditions in Mice
- PMID: 34047195
- PMCID: PMC8165871
- DOI: 10.1177/10760296211018510
Slounase, a Batroxobin Containing Activated Factor X Effectively Enhances Hemostatic Clot Formation and Reducing Bleeding in Hypocoagulant Conditions in Mice
Abstract
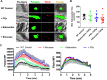
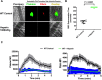
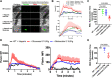
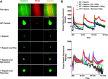
Uncontrolled bleeding associated with trauma and surgery is the leading cause of preventable death. Batroxobin, a snake venom-derived thrombin-like serine protease, has been shown to clot fibrinogen by cleaving fibrinopeptide A in a manner distinctly different from thrombin, even in the presence of heparin. The biochemical properties of batroxobin and its effect on coagulation have been well characterized in vitro. However, the efficacy of batroxobin on hemostatic clot formation in vivo is not well studied due to the lack of reliable in vivo hemostasis models. Here, we studied the efficacy of batroxobin and slounase, a batroxobin containing activated factor X, on hemostatic clot composition and bleeding using intravital microcopy laser ablation hemostasis models in micro and macro vessels and liver puncture hemostasis models in normal and heparin-induced hypocoagulant mice. We found that prophylactic treatment in wild-type mice with batroxobin, slounase and activated factor X significantly enhanced platelet-rich fibrin clot formation following vascular injury. In heparin-treated mice, batroxobin treatment resulted in detectable fibrin formation and a modest increase in hemostatic clot size, while activated factor X had no effect. In contrast, slounase treatment significantly enhanced both platelet recruitment and fibrin formation, forming a stable clot and shortening bleeding time and blood loss in wild-type and heparin-treated hypocoagulant mice. Our data demonstrate that, while batroxobin enhances fibrin formation, slounase was able to enhance hemostasis in normal mice and restore hemostasis in hypocoagulant conditions via the enhancement of fibrin formation and platelet activation, indicating that slounase is more effective in controlling hemorrhage.
Keywords: batroxobin; bleeding; coagulation; fibrin; hemostasis; platelet.
Conflict of interest statement
Figures

Similar articles
-
The effective control of a bleeding injury using a medical adhesive containing batroxobin.Biomed Mater. 2014 Apr;9(2):025002. doi: 10.1088/1748-6041/9/2/025002. Epub 2014 Jan 31. Biomed Mater. 2014. PMID: 24487019
-
Functional improvement of hemostatic dressing by addition of recombinant batroxobin.Acta Biomater. 2017 Jan 15;48:175-185. doi: 10.1016/j.actbio.2016.10.024. Epub 2016 Oct 18. Acta Biomater. 2017. PMID: 27769944
-
Perioperative monitoring of primary and secondary hemostasis in coronary artery bypass grafting.Semin Thromb Hemost. 2005;31(4):426-40. doi: 10.1055/s-2005-916678. Semin Thromb Hemost. 2005. PMID: 16149021
-
Potential role of recombinant factor VIIa as a hemostatic agent.Clin Adv Hematol Oncol. 2003 Feb;1(2):112-9. Clin Adv Hematol Oncol. 2003. PMID: 16224390 Review.
-
Defibrinogenating enzymes.Drugs. 1997;54 Suppl 3:18-30; discussion 30-1. doi: 10.2165/00003495-199700543-00005. Drugs. 1997. PMID: 9360849 Review.
Cited by
-
Smart thrombosis inhibitors without bleeding side effects via charge tunable ligand design.Nat Commun. 2023 Apr 26;14(1):2177. doi: 10.1038/s41467-023-37709-0. Nat Commun. 2023. PMID: 37100783 Free PMC article.
References
-
- Kauvar DS, Lefering R, Wade CE. Impact of hemorrhage on trauma outcome: an overview of epidemiology, clinical presentations, and therapeutic considerations. J Trauma. 2006;60(6 Suppl):S3–11. - PubMed
-
- Ruggeri ZM. Platelets in atherothrombosis. Nat Med. 2002;8(11):1227–1234. - PubMed
-
- Ni H, Freedman J. Platelets in hemostasis and thrombosis: role of integrins and their ligands. Transfus Apher Sci. 2003;28(3):257–264. - PubMed
-
- Jackson SP. The growing complexity of platelet aggregation. Blood. 2007;109(12):5087–5095. - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

