Molecular mechanisms of anthracycline cardiovascular toxicity
- PMID: 34047339
- PMCID: PMC10866014
- DOI: 10.1042/CS20200301
Molecular mechanisms of anthracycline cardiovascular toxicity
Abstract
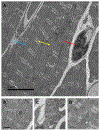
Anthracyclines are effective chemotherapeutic agents, commonly used in the treatment of a variety of hematologic malignancies and solid tumors. However, their use is associated with a significant risk of cardiovascular toxicities and may result in cardiomyopathy and heart failure. Cardiomyocyte toxicity occurs via multiple molecular mechanisms, including topoisomerase II-mediated DNA double-strand breaks and reactive oxygen species (ROS) formation via effects on the mitochondrial electron transport chain, NADPH oxidases (NOXs), and nitric oxide synthases (NOSs). Excess ROS may cause mitochondrial dysfunction, endoplasmic reticulum stress, calcium release, and DNA damage, which may result in cardiomyocyte dysfunction or cell death. These pathophysiologic mechanisms cause tissue-level manifestations, including characteristic histopathologic changes (myocyte vacuolization, myofibrillar loss, and cell death), atrophy and fibrosis, and organ-level manifestations including cardiac contractile dysfunction and vascular dysfunction. In addition, these mechanisms are relevant to current and emerging strategies to diagnose, prevent, and treat anthracycline-induced cardiomyopathy. This review details the established and emerging data regarding the molecular mechanisms of anthracycline-induced cardiovascular toxicity.
Keywords: anthracycline; cardiomyopathy; cardiotoxicity; reactive oxygen species; topoisomerases.
© 2021 The Author(s). Published by Portland Press Limited on behalf of the Biochemical Society.
Conflict of interest statement
Dr. Zemljic-Harpf receives funding from the Merck Investigator Studies Program and Scientific Engagements.
Figures


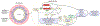
References
-
- Mulrooney DA, Hyun G, Ness KK, Ehrhardt MJ, Yasui Y, Duprez D, Howell RM, Leisenring WM, Constine LS, Tonorezos E, et al. (2020) Major cardiac events for adult survivors of childhood cancer diagnosed between 1970 and 1999: report from the Childhood Cancer Survivor Study cohort. The BMJ 368. - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

