Establishing functional lentiviral vector production in a stirred bioreactor for CAR-T cell therapy
- PMID: 34047682
- PMCID: PMC8806440
- DOI: 10.1080/21655979.2021.1931644
Establishing functional lentiviral vector production in a stirred bioreactor for CAR-T cell therapy
Abstract
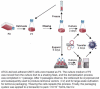
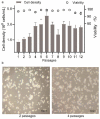
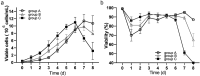
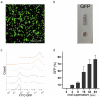
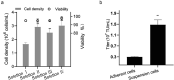
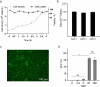
As gene delivery tools, lentiviral vectors (LV) have broad applications in chimeric antigen receptor therapy (CAR-T). Large-scale production of functional LV is limited by the adherent, serum-dependent nature of HEK293T cells used in the manufacturing. HEK293T adherent cells were adapted to suspension cells in a serum-free medium to establish large-scale processes for functional LV production in a stirred bioreactor without micro-carriers. The results showed that 293 T suspension was successfully cultivated in F media (293 CD05 medium and SMM293-TII with 1:1 volume ratio), and the cells retained the capacity for LV production. After cultivation in a 5.5 L bioreactor for 4 days, the cells produced 1.5 ± 0.3 × 107 TU/mL raw LV, and the lentiviral transduction efficiency was 48.6 ± 2.8% in T Cells. The yield of LV equaled to the previous shake flask. The critical process steps were completed to enable a large-scale LV production process. Besides, a cryopreservation solution was developed to reduce protein involvement, avoid cell grafting and reduce process cost. The process is cost-effective and easy to scale up production, which is expected to be highly competitive.
Keywords: CAR-T cell therapy; Functional; HEK293T cells; lentiviral vectors; stirred bioreactors.
Figures
References
-
- McCarron A, Donnelley M, McIntyre C, et al. Challenges of up-scaling lentivirus production and processing. J Biotechnol. 2016;240:23–30. - PubMed
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
