Difference in SARS-CoV-2 Antibody Status Between Patients With Cancer and Health Care Workers During the COVID-19 Pandemic in Japan
- PMID: 34047762
- PMCID: PMC8164151
- DOI: 10.1001/jamaoncol.2021.2159
Difference in SARS-CoV-2 Antibody Status Between Patients With Cancer and Health Care Workers During the COVID-19 Pandemic in Japan
Abstract
Importance: Patients with cancer and health care workers (HCWs) are at high risk of SARS-CoV-2 infection. Assessing the antibody status of patients with cancer and HCWs can help understand the spread of COVID-19 in cancer care.
Objective: To evaluate serum SARS-CoV-2 antibody status in patients with cancer and HCWs during the COVID-19 pandemic in Japan.
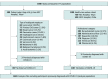
Design, setting, and participants: Participants were enrolled for this prospective cross-sectional study between August 3 and October 30, 2020, from 2 comprehensive cancer centers in the epidemic area around Tokyo, Japan. Patients with cancer aged 16 years or older and employees were enrolled. Participants with suspected COVID-19 infection at the time of enrollment were excluded.
Exposures: Cancer of any type and cancer treatment, including chemotherapy, surgery, immune checkpoint inhibitors, radiotherapy, and targeted molecular therapy.
Main outcomes and measures: Seroprevalence and antibody levels in patients with cancer and HCWs. Seropositivity was defined as positivity to nucleocapsid IgG (N-IgG) and/or spike IgG (S-IgG). Serum levels of SARS-CoV-2 IgM and IgG antibodies against the nucleocapsid and spike proteins were measured by chemiluminescent enzyme immunoassay.
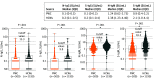
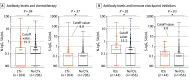
Results: A total of 500 patients with cancer (median age, 62.5 years [range, 21-88 years]; 265 men [55.4%]) and 1190 HCWs (median age, 40 years [range, 20-70 years]; 382 men [25.4%]) were enrolled. In patients with cancer, 489 (97.8%) had solid tumors, and 355 (71.0%) had received anticancer treatment within 1 month. Among HCWs, 385 (32.3%) were nurses or assistant nurses, 266 (22.4%) were administrative officers, 197 (16.6%) were researchers, 179 (15.0%) were physicians, 113 (9.5%) were technicians, and 50 (4.2%) were pharmacists. The seroprevalence was 1.0% (95% CI, 0.33%-2.32%) in patients and 0.67% (95% CI, 0.29%-1.32%) in HCWs (P = .48). However, the N-IgG and S-IgG antibody levels were significantly lower in patients than in HCWs (N-IgG: β, -0.38; 95% CI, -0.55 to -0.21; P < .001; and S-IgG: β, -0.39; 95% CI, -0.54 to -0.23; P < .001). Additionally, among patients, N-IgG levels were significantly lower in those who received chemotherapy than in those who did not (median N-IgG levels, 0.1 [interquartile range (IQR), 0-0.3] vs 0.1 [IQR, 0-0.4], P = .04). In contrast, N-IgG and S-IgG levels were significantly higher in patients who received immune checkpoint inhibitors than in those who did not (median N-IgG levels: 0.2 [IQR, 0.1-0.5] vs 0.1 [IQR, 0-0.3], P = .02; S-IgG levels: 0.15 [IQR, 0-0.3] vs 0.1[IQR, 0-0.2], P = .02).
Conclusions and relevance: In this cross-sectional study of Japanese patients with cancer and HCWs, the seroprevalence of SARS-CoV-2 antibodies did not differ between the 2 groups; however, findings suggest that comorbid cancer and treatment with systemic therapy, including chemotherapy and immune checkpoint inhibitors, may influence the immune response to SARS-CoV-2.
Conflict of interest statement
Figures
Comment in
-
Immune Responses to SARS-CoV-2 Among Patients With Cancer: What Can Seropositivity Tell Us?JAMA Oncol. 2021 Aug 1;7(8):1123-1125. doi: 10.1001/jamaoncol.2021.2096. JAMA Oncol. 2021. PMID: 34047766 No abstract available.
References
-
- WHO . Coronavirus Disease (COVID-19) Dashboard 2021. Accessed February 1, 2021. https://covid19.who.int
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous

