Evaluation of Seropositivity Following BNT162b2 Messenger RNA Vaccination for SARS-CoV-2 in Patients Undergoing Treatment for Cancer
- PMID: 34047765
- PMCID: PMC8164144
- DOI: 10.1001/jamaoncol.2021.2155
Evaluation of Seropositivity Following BNT162b2 Messenger RNA Vaccination for SARS-CoV-2 in Patients Undergoing Treatment for Cancer
Abstract
Importance: Patients with cancer undergoing treatment are at high risk of COVID-19 following SARS-CoV-2 infection; however, their ability to produce an adequate antibody response to messenger RNA SARS-CoV-2 vaccines is unclear.
Objective: To evaluate rates of antispike (anti-S) antibody response to a BNT162b2 vaccine in patients with cancer who are undergoing systemic treatment vs healthy controls.
Design, setting, and participants: This prospective cohort study included 102 adult patients with solid tumors undergoing active intravenous anticancer treatment and 78 controls who received the second dose of the BNT162b2 vaccine at least 12 days before enrollment. The controls were taken from a convenience sample of the patients' family/caregivers who accompanied them to treatment. The study was conducted between February 22, 2021, and March 15, 2021 at Davidoff Cancer Center at Beilinson Hospital (Petah Tikva, Israel).
Interventions: Blood samples were drawn from the study participants. Serum samples were analyzed and the titers of the IgG antibodies against SARS-CoV-2 spike receptor-binding domain were determined using a commercially available immunoassay. Seropositivity was defined as 50 or greater AU/mL.
Main outcomes and measures: The primary outcome was the rate of seropositivity. Secondary outcomes included comparisons of IgG titers and identifying factors that were associated with seropositivity using univariate/multivariable analyses.
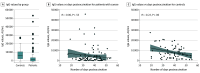
Results: The analysis included 180 participants, which comprised 102 patients with cancer (median [interquartile range (IQR)] age, 66 [56-72] years; 58 men [57%]) and 78 healthy controls (median [IQR] age, 62 [49-70] years; 25 men [32%]). The most common tumor type was gastrointestinal (29 [28%]). In the patient group, 92 (90%) were seropositive for SARS-CoV 2 antispike IgG antibodies after the second vaccine dose, whereas in the control group, all were seropositive. The median IgG titer in the patients with cancer was significantly lower than that in the controls (1931 [IQR, 509-4386] AU/mL vs 7160 [IQR, 3129-11 241] AU/mL; P < .001). In a multivariable analysis, the only variable that was significantly associated with lower IgG titers was treatment with chemotherapy plus immunotherapy (β, -3.5; 95% CI, -5.6 to -1.5).
Conclusions and relevance: In this cohort study of patients with cancer who were receiving active systemic therapy, 90% of patients exhibited adequate antibody response to the BNT162b2 vaccine, although their antibody titers were significantly lower than those of healthy controls. Further research into the clinical relevance of lower titers and their durability is required. Nonetheless, the data support vaccinating patients with cancer as a high priority, even during therapy.
Conflict of interest statement
Figures
Comment in
-
Immune Responses to SARS-CoV-2 Among Patients With Cancer: What Can Seropositivity Tell Us?JAMA Oncol. 2021 Aug 1;7(8):1123-1125. doi: 10.1001/jamaoncol.2021.2096. JAMA Oncol. 2021. PMID: 34047766 No abstract available.
-
Durability of Response to SARS-CoV-2 BNT162b2 Vaccination in Patients on Active Anticancer Treatment.JAMA Oncol. 2021 Nov 1;7(11):1716-1718. doi: 10.1001/jamaoncol.2021.4390. JAMA Oncol. 2021. PMID: 34379092 Free PMC article.
References
-
- Worldometer . Coronavirus update (live): 123,438,047 cases and 2,722,111 deaths from COVID-19 virus pandemic. Accessed March 21, 2021. https://www.worldometers.info/coronavirus
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous