A Centralized Program with Stepped Support Increases Adherence to Colorectal Cancer Screening Over 9 Years: a Randomized Trial
- PMID: 34047921
- PMCID: PMC8162159
- DOI: 10.1007/s11606-021-06922-2
A Centralized Program with Stepped Support Increases Adherence to Colorectal Cancer Screening Over 9 Years: a Randomized Trial
Abstract
Background: Screening over many years is required to optimize colorectal cancer (CRC) outcomes.
Objective: To evaluate the effect of a CRC screening intervention on adherence to CRC screening over 9 years.
Design: Randomized trial.
Setting: Integrated health care system in Washington state.
Participants: Between August 2008 and November 2009, 4653 adults in a Washington state integrated health care system aged 50-74 due for CRC screening were randomized to usual care (UC; N =1163) or UC plus study interventions (interventions: N = 3490).
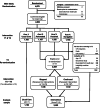
Interventions: Years 1 and 2: (arm 1) UC or this plus study interventions; (arm 2) mailed fecal tests or information on scheduling colonoscopy; (arm 3) mailings plus brief telephone assistance; or (arm 4) mailings and assistance plus nurse navigation. In year 3, stepped-intensity participants (arms 2, 3, and 4 combined) still eligible for screening were randomized to either stopped or continued interventions in years 3 and 5-9.
Main measures: Time in adherence to CRC testing over 9 years (covered time, primary outcome), and percent with no CRC testing in participants assigned to any intervention compared to UC only. Poisson regression models estimated incidence rate ratios for covered time, adjusting for patient characteristics and accounting for variable follow-up time.
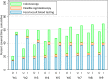
Key results: Compared to UC, intervention participants had 21% more covered time over 9 years (57.5% vs. 69.1%; adjusted incidence rate ratio 1.21, 95% confidence interval 1.16-1.25, P<0.001). Fecal testing accounted for almost all additional covered time among intervention patients. Compared to UC, intervention participants were also more likely to have completed at least one CRC screening test over 9 years or until censorship (88.6% vs. 80.6%, P<0.001).
Conclusions: An outreach program that included mailed fecal tests and phone follow-up led to increased adherence to CRC testing and fewer age-eligible individuals without any CRC testing over 9 years.
Trial registration: Systems of Support (SOS) to Increase Colon Cancer Screening and Follow-up (SOS), NCT00697047, clinicaltrials.gov/ct2/show/NCT00697047.
Keywords: colorectal cancer; health care system; mailed fecal tests; randomized trial; screening.
© 2021. Society of General Internal Medicine.
Figures
References
-
- Siegel RL, Miller KD, Goding Sauer A, et al. Colorectal cancer statistics, 2020. CA Cancer J Clin. 2020;70(3):145–164. - PubMed
-
- American Cancer Society. Cancer facts & figures 2021. 2021; https://www.cancer.org/research/cancer-facts-statistics/all-cancer-facts.... Accessed April 14, 2021.
-
- Freedman JD, Mitchell CK. A simple strategy to improve patient adherence to outpatient fecal occult blood testing. J Gen Intern Med. 1994;9(8):462–464. - PubMed
-
- Church TR, Yeazel MW, Jones RM, et al. A randomized trial of direct mailing of fecal occult blood tests to increase colorectal cancer screening. J Natl Cancer Inst. 2004;96(10):770–780. - PubMed
-
- Coronado GD, Golovaty I, Longton G, Levy L, Jimenez R. Effectiveness of a clinic-based colorectal cancer screening promotion program for underserved Hispanics. Cancer. 2011;117(8):1745–1754. - PubMed
Publication types
MeSH terms
Associated data
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

