Cecal Slurry Injection in Neonatal and Adult Mice
- PMID: 34048005
- PMCID: PMC8482797
- DOI: 10.1007/978-1-0716-1488-4_4
Cecal Slurry Injection in Neonatal and Adult Mice
Abstract
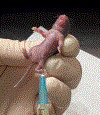
Studying the pathophysiology of sepsis still requires animal models, and the mouse remains the most commonly used species. Here we discuss the "cecal slurry" (CS) model of polymicrobial, peritoneal sepsis and compare and contrast it to other commonly used methods. Among the different murine models of sepsis, cecal ligation and puncture (CLP), and not the CS, is often considered the "gold standard" to induce polymicrobial sepsis in laboratory animals. CLP is a well-described model involving a simple surgical procedure that closely mimics the clinical course of intra-abdominal sepsis. However, CLP may not be an option for experiments involving newborn pups, where the cecum is indistinguishable from small bowel, where differences in microbiome content may affect the experiment, or where surgical procedures/anesthesia exposure needs to be limited. An important alternative method is the CS model, involving the intraperitoneal injection of cecal contents from a donor animal into the peritoneal cavity of a recipient animal to induce polymicrobial sepsis. Furthermore, CS is an effective alternative model of intraperitoneal polymicrobial sepsis in adult mice and can now be considered the "gold standard" for experiments in neonatal mice.
Keywords: Cecal slurry; Infection; Murine sepsis; Neonatal sepsis; Polymicrobial sepsis; Sepsis.
Figures


References
-
- Cohen J, Vincent JL, Adhikari NK, Machado FR, Angus DC, Calandra T, et al.Sepsis: a roadmap for future research. The Lancet Infectious diseases. 2015;15(5):581–614. - PubMed
-
- Shane AL, Sanchez PJ, Stoll BJ. Neonatal sepsis. Lancet (London, England). 2017;390(10104):1770–80. - PubMed
-
- Fleischmann C, Scherag A, Adhikari NK, Hartog CS, Tsaganos T, Schlattmann P, et al.Assessment of Global Incidence and Mortality of Hospital-treated Sepsis. Current Estimates and Limitations. American journal of respiratory and critical care medicine. 2016;193(3):259–72. - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

