MRI-Based Iron Phenotyping and Patient Selection for Next-Generation Sequencing of Non-Homeostatic Iron Regulator Hemochromatosis Genes
- PMID: 34048062
- PMCID: PMC8596846
- DOI: 10.1002/hep.31982
MRI-Based Iron Phenotyping and Patient Selection for Next-Generation Sequencing of Non-Homeostatic Iron Regulator Hemochromatosis Genes
Abstract
Background and aims: High serum ferritin is frequent among patients with chronic liver disease and commonly associated with hepatic iron overload. Genetic causes of high liver iron include homozygosity for the p.Cys282Tyr variant in homeostatic iron regulator (HFE) and rare variants in non-HFE genes. The aims of the present study were to describe the landscape and frequency of mutations in hemochromatosis genes and determine whether patient selection by noninvasive hepatic iron quantification using MRI improves the diagnostic yield of next-generation sequencing (NGS) in patients with hyperferritinemia.
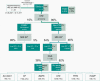
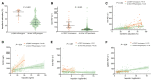
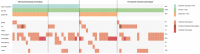
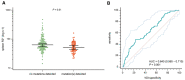
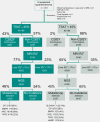
Approach and results: A cohort of 410 unselected liver clinic patients with high serum ferritin (defined as ≥200 μg/L for women and ≥300 μg/L for men) was investigated by HFE genotyping and abdominal MRI R2*. Forty-one (10%) patients were homozygous for the p.Cys282Tyr variant in HFE. Of the remaining 369 patients, 256 (69%) had high transferrin saturation (TSAT; ≥45%) and 199 (53%) had confirmed hepatic iron overload (liver R2* ≥70 s-1 ). NGS of hemochromatosis genes was carried out in 180 patients with hepatic iron overload, and likely pathogenic variants were identified in 68 of 180 (38%) patients, mainly in HFE (79%), ceruloplasmin (25%), and transferrin receptor 2 (19%). Low spleen iron (R2* <50 s-1 ), but not TSAT, was significantly associated with the presence of mutations. In 167 patients (93%), no monogenic cause of hepatic iron overload could be identified.
Conclusions: In patients without homozygosity for p.Cys282Tyr, coincident pathogenic variants in HFE and non-HFE genes could explain hyperferritinemia with hepatic iron overload in a subset of patients. Unlike HFE hemochromatosis, this type of polygenic hepatic iron overload presents with variable TSAT. High ferritin in blood is an indicator of the iron storage disease, hemochromatosis. A simple genetic test establishes this diagnosis in the majority of patients affected. MRI of the abdomen can guide further genetic testing.
© 2021 The Authors. Hepatology published by Wiley Periodicals LLC on behalf of American Association for the Study of Liver Diseases.
Figures
Similar articles
-
Re-evaluating the utility of iron indices in hereditary hemochromatosis genotyping: A retrospective study.Clin Biochem. 2025 Jan;135:110860. doi: 10.1016/j.clinbiochem.2024.110860. Epub 2024 Nov 29. Clin Biochem. 2025. PMID: 39617311
-
HFE genotype in patients with hemochromatosis and other liver diseases.Ann Intern Med. 1999 Jun 15;130(12):953-62. doi: 10.7326/0003-4819-130-12-199906150-00002. Ann Intern Med. 1999. PMID: 10383365
-
HFE Genotype, Ferritin Levels and Transferrin Saturation in Patients with Suspected Hereditary Hemochromatosis.Genes (Basel). 2021 Jul 28;12(8):1162. doi: 10.3390/genes12081162. Genes (Basel). 2021. PMID: 34440336 Free PMC article.
-
[Pathophysiology and genetics of classic HFE (type 1) hemochromatosis].Presse Med. 2007 Sep;36(9 Pt 2):1271-7. doi: 10.1016/j.lpm.2007.03.038. Epub 2007 May 22. Presse Med. 2007. PMID: 17521857 Review. French.
-
A diagnostic approach to hyperferritinemia with a non-elevated transferrin saturation.J Hepatol. 2011 Aug;55(2):453-8. doi: 10.1016/j.jhep.2011.02.010. Epub 2011 Feb 24. J Hepatol. 2011. PMID: 21354228 Review.
Cited by
-
Iron as a therapeutic target in chronic liver disease.World J Gastroenterol. 2023 Jan 28;29(4):616-655. doi: 10.3748/wjg.v29.i4.616. World J Gastroenterol. 2023. PMID: 36742167 Free PMC article. Review.
-
Iron and Liver Disease.Adv Exp Med Biol. 2025;1480:237-252. doi: 10.1007/978-3-031-92033-2_16. Adv Exp Med Biol. 2025. PMID: 40603795 Review.
References
-
- Camaschella C. Iron deficiency. Blood 2019;133:30‐39. - PubMed
-
- Allen KJ, Gurrin LC, Constantine CC, Osborne NJ, Delatycki MB, Nicoll AJ, et al. Iron‐overload‐related disease in HFE hereditary hemochromatosis. N Engl J Med 2008;358:221‐230. - PubMed
-
- Koperdanova M, Cullis JO. Interpreting raised serum ferritin levels. BMJ 2015;351:h3692. - PubMed
-
- Moirand R, Mortaji AM, Loreal O, Paillard F, Brissot P, Deugnier Y. A new syndrome of liver iron overload with normal transferrin saturation. Lancet 1997;349:95‐97. - PubMed
-
- Whitfield JB, Zhu G, Heath AC, Powell LW, Martin NG. Effects of alcohol consumption on indices of iron stores and of iron stores on alcohol intake markers. Alcohol Clin Exp Res 2001;25:1037‐1045. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

