Gemtuzumab Ozogamicin Improves Event-Free Survival and Reduces Relapse in Pediatric KMT2A-Rearranged AML: Results From the Phase III Children's Oncology Group Trial AAML0531
- PMID: 34048275
- PMCID: PMC8478392
- DOI: 10.1200/JCO.20.03048
Gemtuzumab Ozogamicin Improves Event-Free Survival and Reduces Relapse in Pediatric KMT2A-Rearranged AML: Results From the Phase III Children's Oncology Group Trial AAML0531
Abstract
Purpose: We investigated the impact of the CD33-targeted agent gemtuzumab ozogamicin (GO) on survival in pediatric patients with KMT2A-rearranged (KMT2A-r) acute myeloid leukemia (AML) enrolled in the Children's Oncology Group trial AAML0531 (NCT01407757).
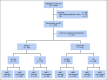
Methods: Patients with KMT2A-r AML were identified and clinical characteristics described. Five-year overall survival (OS), event-free survival (EFS), disease-free survival (DFS), and relapse risk (RR) were determined overall and for higher-risk versus not high-risk translocation partners. GO's impact on response was determined and outcomes based on consolidation approach (hematopoietic stem cell transplant [HSCT] v chemotherapy) described.
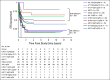
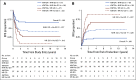
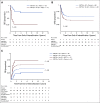
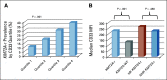
Results: Two hundred fifteen (21%) of 1,022 patients enrolled had KMT2A-r AML. Five-year EFS and OS from study entry were 38% and 58%, respectively. EFS was superior with GO treatment (EFS 48% with GO v 29% without, P = .003), although OS was comparable (63% v 53%, P = .054). For patients with KMT2A-r AML who achieved complete remission, GO was associated with lower RR (40% GO v 66% patients who did not receive GO [No-GO], P = .001) and improved 5-year DFS (GO 57% v No-GO 33%, P = .002). GO benefit was observed in both higher-risk and not high-risk KMT2A-r subsets. For patients who underwent HSCT, prior GO exposure was associated with decreased relapse (5-year RR: 28% GO and HSCT v 73% No-GO and HSCT, P = .006). In multivariable analysis, GO was independently associated with improved EFS, improved DFS, and reduced RR.
Conclusion: GO added to conventional chemotherapy improved outcomes for KMT2A-r AML; consolidation with HSCT may further enhance outcomes. Future clinical trials should study CD33-targeted agents in combination with HSCT for pediatric KMT2A-r AML.
Conflict of interest statement
Figures

References
-
- Harrison CJ, Hills RK, Moorman AV, et al. : Cytogenetics of childhood acute myeloid leukemia: United Kingdom Medical Research Council Treatment trials AML 10 and 12. J Clin Oncol 28:2674-2681, 2010 - PubMed
Publication types
MeSH terms
Substances
Associated data
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

