Effectiveness of Adding a Large Dose of Shoulder Strengthening to Current Nonoperative Care for Subacromial Impingement: A Pragmatic, Double-Blind Randomized Controlled Trial (SExSI Trial)
- PMID: 34048281
- PMCID: PMC8411479
- DOI: 10.1177/03635465211016008
Effectiveness of Adding a Large Dose of Shoulder Strengthening to Current Nonoperative Care for Subacromial Impingement: A Pragmatic, Double-Blind Randomized Controlled Trial (SExSI Trial)
Abstract
Background: A strong recommendation against subacromial decompression surgery was issued in 2019. This leaves nonoperative care as the only treatment option, but recent studies suggest that the dose of strengthening exercise is not sufficient in current nonoperative care. At this point, it is unknown if adding more strengthening to current nonoperative care is of clinical value.
Purpose: To assess the effectiveness of adding a large dose of shoulder strengthening to current nonoperative care for subacromial impingement compared with usual care alone.
Study design: Randomized controlled trial; Level of evidence, 1.
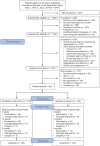
Methods: In this double-blinded, pragmatic randomized controlled trial, we randomly allocated 200 consecutive patients referred to orthopaedic shoulder specialist care for long-standing shoulder pain (>3 months), aged 18 to 65 years and diagnosed with subacromial impingement using validated criteria, to the intervention group (IG) or control group (CG). Outcome assessors were blinded, and participants were blinded to the study hypothesis as well as to the treatment method in the other group. The CG received usual nonoperative care; the IG underwent the same plus an add-on intervention designed to at least double the total dose of shoulder strengthening. The primary outcome was the Shoulder Pain and Disability Index (SPADI; 0-100) at 4-month follow-up, with 10 points defined as the minimal clinically important difference. Secondary outcomes included shoulder strength, range of motion, health-related quality of life, and the Patient Acceptable Symptom State (PASS).
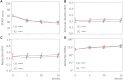
Results: Intention-to-treat and per-protocol analyses showed no significant or clinically relevant between-group differences for any outcome. From baseline to 4-month follow-up, SPADI scores improved in both groups (intention-to-treat analysis; IG, -22.1 points; CG, -22.7 points; between-group mean difference, 0.6 points [95% CI, -5.5 to 6.6]). At 4 months after randomization, only 54% of the IG and 48% of the CG (P = .4127) reached the PASS. No serious adverse events were reported.
Conclusion: Adding a large dose of shoulder strengthening to current nonoperative care for patients with subacromial impingement did not result in superior shoulder-specific patient-reported outcomes. Moreover, approximately half of all randomized patients did not achieve the PASS after 4 months of nonoperative care, leaving many of these patients with unacceptable symptoms. This study showed that adding more exercise is not a viable solution to this problem.
Registration: NCT02747251 (ClinicalTrials.gov identifier).
Keywords: physical therapy; randomized controlled trial; resistance training; rotator cuff; shoulder pain.
Conflict of interest statement
One or more of the authors has declared the following potential conflict of interest or source of funding: This study was supported by grants from Fysioterapipraksisfonden and grants from the Danish Rheumatism Association throughout the duration of the study. T.B. has received speaking fees from Zimmer Biomet and Novartis. AOSSM checks author disclosures against the Open Payments Database (OPD). AOSSM has not conducted an independent investigation on the OPD and disclaims any liability or responsibility relating thereto.
Figures
Comment in
-
Effectiveness of Adding a Large Dose of Shoulder Strengthening to Current Nonoperative Care for Subacromial Impingement: A Pragmatic, Double-Blind Randomized Controlled Trial (SExSI Trial): Response.Am J Sports Med. 2022 Mar;50(3):NP20-NP23. doi: 10.1177/03635465211055449. Am J Sports Med. 2022. PMID: 35289223 No abstract available.
-
Effectiveness of Adding a Large Dose of Shoulder Strengthening to Current Nonoperative Care for Subacromial Impingement: A Pragmatic, Double-Blind Randomized Controlled Trial (SExSI Trial): Letter to the Editor.Am J Sports Med. 2022 Mar;50(3):NP18-NP19. doi: 10.1177/03635465211055452. Am J Sports Med. 2022. PMID: 35289230 No abstract available.
Similar articles
-
The Strengthening Exercises in Shoulder Impingement trial (The SExSI-trial) investigating the effectiveness of a simple add-on shoulder strengthening exercise programme in patients with long-lasting subacromial impingement syndrome: Study protocol for a pragmatic, assessor blinded, parallel-group, randomised, controlled trial.Trials. 2018 Mar 2;19(1):154. doi: 10.1186/s13063-018-2509-7. Trials. 2018. PMID: 29499710 Free PMC article.
-
Level of pain catastrophising determines if patients with long-standing subacromial impingement benefit from more resistance exercise: predefined secondary analyses from a pragmatic randomised controlled trial (the SExSI Trial).Br J Sports Med. 2023 Jul;57(13):842-848. doi: 10.1136/bjsports-2022-106383. Epub 2023 Mar 10. Br J Sports Med. 2023. PMID: 36898767 Free PMC article. Clinical Trial.
-
Effectiveness of Adding a Large Dose of Shoulder Strengthening to Current Nonoperative Care for Subacromial Impingement: A Pragmatic, Double-Blind Randomized Controlled Trial (SExSI Trial): Response.Am J Sports Med. 2022 Mar;50(3):NP20-NP23. doi: 10.1177/03635465211055449. Am J Sports Med. 2022. PMID: 35289223 No abstract available.
-
Effect of supervised physiotherapy versus home exercise program in patients with subacromial impingement syndrome: A systematic review and meta-analysis.Phys Ther Sport. 2020 Jan;41:34-42. doi: 10.1016/j.ptsp.2019.11.003. Epub 2019 Nov 6. Phys Ther Sport. 2020. PMID: 31726386
-
Effectiveness of Therapeutical Interventions on the Scapulothoracic Complex in the Management of Patients with Subacromial Impingement and Frozen Shoulder: A Systematic Review.J Funct Morphol Kinesiol. 2023 Mar 27;8(2):38. doi: 10.3390/jfmk8020038. J Funct Morphol Kinesiol. 2023. PMID: 37092370 Free PMC article. Review.
Cited by
-
What are the predictors of response to physiotherapy in patients with massive irreparable rotator cuff tears? Gaining expert consensus using an international e-Delphi study.BMC Musculoskelet Disord. 2024 Oct 12;25(1):807. doi: 10.1186/s12891-024-07872-6. BMC Musculoskelet Disord. 2024. PMID: 39395963 Free PMC article.
-
Painful considerations in exercise-management for rotator cuff related shoulder pain: a scoping review on pain-related prescription parameters.BMC Musculoskelet Disord. 2025 Feb 22;26(1):180. doi: 10.1186/s12891-025-08411-7. BMC Musculoskelet Disord. 2025. PMID: 39987051 Free PMC article.
-
Short-term effectiveness of high-load compared with low-load strengthening exercise on self-reported function in patients with hypermobile shoulders: a randomised controlled trial.Br J Sports Med. 2022 Jun 1;56(22):1269-76. doi: 10.1136/bjsports-2021-105223. Online ahead of print. Br J Sports Med. 2022. PMID: 35649707 Free PMC article.
-
The influence of exercise on clinical pain and pain mechanisms in patients with subacromial pain syndrome.Eur J Pain. 2022 Oct;26(9):1882-1895. doi: 10.1002/ejp.2010. Epub 2022 Jul 27. Eur J Pain. 2022. PMID: 35852027 Free PMC article.
-
Writing up your clinical trial report for a scientific journal: the REPORT trial guide for effective and transparent research reporting without spin.Br J Sports Med. 2022 Jun;56(12):683-691. doi: 10.1136/bjsports-2021-105058. Epub 2022 Feb 22. Br J Sports Med. 2022. PMID: 35193854 Free PMC article. Review.
References
-
- Braunholtz DA, Edwards SJ, Lilford RJ.Are randomized clinical trials good for us (in the short term)? Evidence for a “trial effect.” J Clin Epidemiol. 2001;54(3):217-224. - PubMed
-
- Cederqvist S, Flinkkilä T, Sormaala M, et al.. Non-surgical and surgical treatments for rotator cuff disease: a pragmatic randomised clinical trial with 2-year follow-up after initial rehabilitation. Ann Rheum Dis. Published online December3, 2020. doi: 10.1136/annrheumdis-2020-219099 - DOI - PMC - PubMed
Publication types
MeSH terms
Associated data
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

