Energy restriction causes metaphase delay and chromosome mis-segregation in cancer cells
- PMID: 34048314
- PMCID: PMC8265792
- DOI: 10.1080/15384101.2021.1930679
Energy restriction causes metaphase delay and chromosome mis-segregation in cancer cells
Abstract
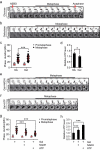
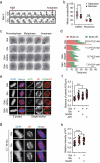
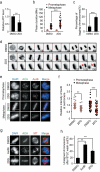
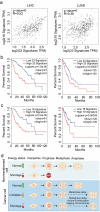
ATP metabolism during mitosis needs to be coordinated with numerous energy-demanding activities, especially in cancer cells whose metabolic pathways are reprogramed to sustain rapid proliferation in a nutrient-deficient environment. Although strategies targeting the energy metabolic pathways have shown therapeutic efficacy in preclinical cancer models, how normal cells and cancer cells differentially respond to energy shortage is unclear. In this study, using time-lapse microscopy, we found that cancer cells displayed unique mitotic phenotypes in a dose-dependent manner upon decreasing ATP (i.e. energy) supply. When reduction in ATP concentration was moderate, chromosome movements in mitosis were barely affected, while the metaphase-anaphase transition was significantly prolonged due to reduced tension between the sister-kinetochores, which delayed the satisfaction of the spindle assembly checkpoint. Further reduction in ATP concentration led to a decreased level of Aurora-B at the centromere, resulting in increased chromosome mis-segregation after metaphase delay. In contrast to cancer cells, ATP restriction in non-transformed cells induced cell cycle arrest in interphase, rather than causing mitotic defects. In addition, data mining of cancer patient database showed a correlation between signatures of energy production and chromosomal instability possibly resulted from mitotic defects. Together, these results reveal that energy restriction induces differential responses in normal and cancer cells, with chromosome mis-segregation only observed in cancer cells. This points to targeting energy metabolism as a potentially cancer-selective therapeutic strategy.
Keywords: ATP; cancer cells; chromosome mis-segregation; energy restriction; metaphase-anaphase transition.
Conflict of interest statement
No potential conflict of interest was reported by the author(s).
Figures
References
-
- Jia L, Kim S, Yu H.. Tracking spindle checkpoint signals from kinetochores to APC/C. Trends Biochem Sci. 2013;38(6):302–311. - PubMed
-
- Skog S, Tribukait B, Sundius G. Energy metabolism and ATP turnover time during the cell cycle of Ehrlich ascites tumour cells. Exp Cell Res. 1982;141:23–29. - PubMed
-
- Salazar-Roa M, Malumbres M. Fueling the cell division cycle. Trends Cell Biol. 2017;27:69–81. - PubMed
-
- Harbauer AB, Opalińska M, Gerbeth C, et al. Mitochondria. Cell cycle-dependent regulation of mitochondrial preprotein translocase. Science. 2014;346:1109–1113. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous
