LncRNA RUSC1-AS1 promotes osteosarcoma progression through regulating the miR-340-5p and PI3K/AKT pathway
- PMID: 34048366
- PMCID: PMC8436931
- DOI: 10.18632/aging.203047
LncRNA RUSC1-AS1 promotes osteosarcoma progression through regulating the miR-340-5p and PI3K/AKT pathway
Abstract
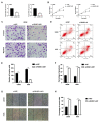
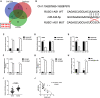
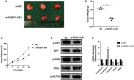
Dysregulation of long noncoding RNA (lncRNA) is frequently involved in the progression and development of osteosarcoma. LncRNA RUSC1-AS1 is reported to be upregulated and acts as an oncogene in hepatocellular carcinoma, cervical cancer and breast cancer. However, its role in osteosarcoma has not been studied yet. In the present study, we investigated the role of RUSC1-AS1 in osteosarcoma both in vitro and in vivo. The results showed that the expression of RUSC1-AS1 was significantly upregulated in osteosarcoma cell line U2OS and HOS compared to that in human osteoblast cell line hFOB1.19. Similar results were found in human samples. Silencing RUSC1-AS1 by siRNA significantly inhibited U2OS and HOS cell proliferation and invasion, measured by CCK-8 and transwell assay. Besides, knockdown of RUSC1-AS1 increased cell apoptosis in osteosarcoma cell lines. In addition, RUSC1-AS1 promoted the epithelial-mesenchymal transition (EMT) process of osteosarcoma cells. In vivo experiments confirmed that RUSC1-AS1 knockdown had an inhibitory effect on osteosarcoma tumor growth. Mechanically, we showed that RUSC1-AS1 directly binds to and inhibits miR-340-5p and activates the PI3K/AKT signaling pathway. In conclusion, our study demonstrated that RUSC1-AS1 promoted osteosarcoma development both in vitro and in vivo through sponging to miR-340-5p and activating the PI3K/AKT signaling pathway. Therefore, RUSC1-AS1 becomes a potential therapeutic target for osteosarcoma.
Keywords: PI3K/AKT; RUSC1-AS1; miR-340-5p; osteosarcoma.
Conflict of interest statement
Figures
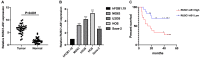



References
-
- Martin-Broto J, Redondo A, Valverde C, Vaz MA, Mora J, Garcia Del Muro X, Gutierrez A, Tous C, Carnero A, Marcilla D, Carranza A, Sancho P, Martinez-Trufero J, et al. Gemcitabine plus sirolimus for relapsed and progressing osteosarcoma patients after standard chemotherapy: a multicenter, single-arm phase II trial of Spanish Group for Research on Sarcoma (GEIS). Ann Oncol. 2017; 28:2994–99. 10.1093/annonc/mdx536 - DOI - PubMed
-
- Sobin LH, Gospodarowicz MK, Wittekind C. TNM classification of malignant tumours. 7th Edition.John Wiley & Sons; 2011.
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

