Avian antibodies (IgY) targeting spike glycoprotein of severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) inhibit receptor binding and viral replication
- PMID: 34048457
- PMCID: PMC8162713
- DOI: 10.1371/journal.pone.0252399
Avian antibodies (IgY) targeting spike glycoprotein of severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) inhibit receptor binding and viral replication
Abstract
Background: The global pandemic of Coronavirus infectious disease 2019 (COVID-19), caused by SARS-CoV-2, has plunged the world into both social and economic disarray, with vaccines still emerging and a continued paucity of personal protective equipment; the pandemic has also highlighted the potential for rapid emergence of aggressive respiratory pathogens and the need for preparedness. Avian immunoglobulins (IgY) have been previously shown in animal models to protect against new infection and mitigate established infection when applied intranasally. We carried out a proof-of-concept study to address the feasibility of using such antibodies as mucosally-applied prophylaxis against SARS-CoV-2.
Methods: Hens were immunized with recombinant S1 spike glycoprotein of the virus, and the resulting IgY was evaluated for binding specificity, inhibition of glycoprotein binding to angiotensin converting enzyme-2 (ACE2) protein (the requisite binding site for the virus), and inhibition of viral replication in Vero cell culture.
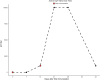
Results: Titers of anti-S1 glycoprotein IgY were evident in yolks at 14 days post-immunization, peaking at 21 days, and at peak concentrations of 16.8 mg/ml. IgY showed strong and significant inhibition of S1/ACE2 binding interactions, and significantly inhibited viral replication at a concentration of 16.8 mg/ml. Four weeks' collection from eggs of two hens produced a total of 1.55 grams of IgY.
Conclusions: In this proof-of-concept study we showed that avian immunoglobulins (IgY) raised against a key virulence factor of the SARS-CoV-2 virus successfully inhibited the critical initial adhesion of viral spike glycoproteins to human ACE2 protein receptors and inhibited viral replication in vitro, in a short period using only two laying hens. We conclude that production of large amounts of IgY inhibiting viral binding and replication of SARS-CoV-2 is feasible, and that incorporation of this or similar material into an intranasal spray and/or other mucosal protecting products may be effective at reducing infection and spread of COVID-19.
Conflict of interest statement
Scaled Microbiomics, LLC (SMB) provided sole funding for this study. Authors CA and JG are employees of SMB; JG is also Founder and CEO of SMB. This does not alter our adherence to PLOS ONE policies on sharing data and materials. No competing interests exist in relation to any authors’ affiliation with Scaled Microbiomics.
Figures




Similar articles
-
Immunoglobulin yolk targeting spike 1, receptor binding domain of spike glycoprotein and nucleocapsid of SARS-CoV-2 blocking RBD-ACE2 binding interaction.Int Immunopharmacol. 2022 Nov;112:109280. doi: 10.1016/j.intimp.2022.109280. Epub 2022 Sep 28. Int Immunopharmacol. 2022. PMID: 36183680 Free PMC article.
-
Generation of Chicken IgY against SARS-COV-2 Spike Protein and Epitope Mapping.J Immunol Res. 2020 Oct 17;2020:9465398. doi: 10.1155/2020/9465398. eCollection 2020. J Immunol Res. 2020. PMID: 33134398 Free PMC article.
-
Egg yolk immunoglobulin (IgY) targeting SARS-CoV-2 S1 as potential virus entry blocker.J Appl Microbiol. 2022 Mar;132(3):2421-2430. doi: 10.1111/jam.15340. Epub 2021 Nov 3. J Appl Microbiol. 2022. PMID: 34706134 Free PMC article.
-
An approach towards development of monoclonal IgY antibodies against SARS CoV-2 spike protein (S) using phage display method: A review.Int Immunopharmacol. 2020 Aug;85:106654. doi: 10.1016/j.intimp.2020.106654. Epub 2020 Jun 3. Int Immunopharmacol. 2020. PMID: 32512271 Free PMC article. Review.
-
Perspectives on development of vaccines against severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2).Hum Vaccin Immunother. 2020 Oct 2;16(10):2366-2369. doi: 10.1080/21645515.2020.1787064. Epub 2020 Sep 22. Hum Vaccin Immunother. 2020. PMID: 32961082 Free PMC article. Review.
Cited by
-
Specific anti-SARS-CoV-2 S1 IgY-scFv is a promising tool for recognition of the virus.AMB Express. 2022 Feb 12;12(1):18. doi: 10.1186/s13568-022-01355-4. AMB Express. 2022. PMID: 35150368 Free PMC article.
-
Affordable IgY-based antiviral prophylaxis for resource-limited settings to address epidemic and pandemic risks.J Glob Health. 2022 Feb 26;12:05009. doi: 10.7189/jogh.12.05009. eCollection 2022. J Glob Health. 2022. PMID: 35265332 Free PMC article.
-
Preclinical Assessment of IgY Antibodies Against Recombinant SARS-CoV-2 RBD Protein for Prophylaxis and Post-Infection Treatment of COVID-19.Front Immunol. 2022 May 10;13:881604. doi: 10.3389/fimmu.2022.881604. eCollection 2022. Front Immunol. 2022. PMID: 35664008 Free PMC article.
-
Evaluation of SARS-CoV-2-Specific IgY Antibodies: Production, Reactivity, and Neutralizing Capability against Virus Variants.Int J Mol Sci. 2024 Jul 21;25(14):7976. doi: 10.3390/ijms25147976. Int J Mol Sci. 2024. PMID: 39063218 Free PMC article.
-
Egg-Derived Anti-SARS-CoV-2 Immunoglobulin Y (IgY) With Broad Variant Activity as Intranasal Prophylaxis Against COVID-19.Front Immunol. 2022 Jun 1;13:899617. doi: 10.3389/fimmu.2022.899617. eCollection 2022. Front Immunol. 2022. PMID: 35720389 Free PMC article. Clinical Trial.
References
-
- Kollberg H, Carlander D, Olesen H, Wejaker PE, Johannesson M, Larsson A. Oral administration of specific yolk antibodies (IgY) may prevent Pseudomonas aeruginosa infections in patients with cystic fibrosis: a phase I feasibility study. Pediatr Pulmonol. 2003;35(6):433–40. Epub 2003/05/15. 10.1002/ppul.10290 . - DOI - PubMed
-
- Patterson R, Youngner JS, Weigle WO, Dixon FJ. Antibody production and transfer to egg yolk in chickens. Journal of immunology (Baltimore, Md: 1950). 1962;89:272–8. Epub 1962/08/01. . - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous

