Validation of a multiplex flow immunoassay for detection of IgG antibodies against SARS-CoV-2 in dried blood spots
- PMID: 34048503
- PMCID: PMC8162624
- DOI: 10.1371/journal.pone.0252621
Validation of a multiplex flow immunoassay for detection of IgG antibodies against SARS-CoV-2 in dried blood spots
Abstract
Background: Dried blood spots (DBS) are an established specimen type for clinical testing given their low cost, ease of collection and storage, and convenient shipping capabilities through the postal system. These attributes are complementary to the expansion of SARS-CoV-2 serologic testing, which may be used to inform community seroprevalence rates.
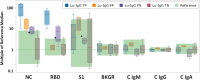
Methods: The Luminex xMAP SARS-CoV-2 Multi-Antigen assay utilizes magnetic beads labeled with three viral antigens (nucleocapsid [NC], receptor binding domain [RBD], spike S1 subunit) to detect anti-viral IgG-class antibodies, and has Food and Drug Administration (FDA) Emergency Use Authorization (EUA) for use in serum and plasma. This assay was modified for use with DBS and validated against paired sera tested by one of two reference assays: the Roche Diagnostics Elecsys anti-SARS-CoV-2 ECLIA or the Euroimmun anti-SARS-CoV-2 IgG ELISA.
Results: 159 paired DBS and serum specimens analyzed using the modified Luminex xMAP assay on DBS and the reference methods on serum showed an overall concordance of 96.9% (154/159). Use of multivariate pattern recognition software (CLIR) for post-analytical interpretation of the Luminex xMAP DBS assay results, instead of manufacturer provided interpretive thresholds, increased overall qualitative result concordance to 99.4% (158/159) between the modified Luminex xMAP DBS and reference results.
Conclusions: Use of DBS for detection of antibodies against SARS-CoV-2 provides comparable results to those obtained using serum. DBS concordance was improved with multivariate pattern recognition software (CLIR). We demonstrate that DBS are a reliable specimen type for SARS-CoV-2 antibody detection using the modified Luminex xMAP assay.
Conflict of interest statement
I have read the journal’s policy and among the authors of this manuscript Dr. Theel has the following broad interests that could be perceived as conflicts of interest: - Consulting fees from Roche Diagnostics and Accelerate Diagnostics - Member of the College of American Pathologists’ Microbiology Resource Committee (travel compensation) - Editor, Clinical Microbiology Newsletter This does not alter our adherence to PLOS ONE policies on sharing data and materials.
Figures

Similar articles
-
Comparison of Dried Blood Spots and Venous Blood for the Detection of SARS-CoV-2 Antibodies in a Population of Nursing Home Residents.Microbiol Spectr. 2021 Oct 31;9(2):e0017821. doi: 10.1128/Spectrum.00178-21. Epub 2021 Sep 22. Microbiol Spectr. 2021. PMID: 34549995 Free PMC article.
-
Detection of SARS-CoV-2 IgG antibodies in dried blood spots.Diagn Microbiol Infect Dis. 2021 Sep;101(1):115425. doi: 10.1016/j.diagmicrobio.2021.115425. Epub 2021 May 13. Diagn Microbiol Infect Dis. 2021. PMID: 34116343 Free PMC article.
-
High-Throughput Multiplex SARS-CoV-2 IgG Microsphere Immunoassay for Dried Blood Spots: A Public Health Strategy for Enhanced Serosurvey Capacity.Microbiol Spectr. 2021 Sep 3;9(1):e0013421. doi: 10.1128/Spectrum.00134-21. Epub 2021 Jul 28. Microbiol Spectr. 2021. PMID: 34319133 Free PMC article.
-
Evaluation of dried blood spots as alternative sampling material for serological detection of anti-SARS-CoV-2 antibodies using established ELISAs.Clin Chem Lab Med. 2021 Jan 8;59(5):979-985. doi: 10.1515/cclm-2020-1436. Print 2021 Apr 27. Clin Chem Lab Med. 2021. PMID: 33554537
-
Use of dried blood spots in the detection of coronavirus disease 2019 (COVID-19): A systematic review.Indian J Med Microbiol. 2024 Sep-Oct;51:100700. doi: 10.1016/j.ijmmb.2024.100700. Epub 2024 Aug 13. Indian J Med Microbiol. 2024. PMID: 39127256
Cited by
-
Detection of IgG Antibodies to SARS-CoV-2 and Neutralizing Capabilities Using the Luminex® xMAP® SARS-CoV-2 Multi-Antigen IgG Assay.Methods Mol Biol. 2022;2511:257-271. doi: 10.1007/978-1-0716-2395-4_19. Methods Mol Biol. 2022. PMID: 35838966
-
SARS-CoV-2 serological findings and exposure risk among employees in school and retail after first and second wave COVID-19 pandemic in Oslo, Norway: a cohort study.Int J Occup Med Environ Health. 2022 Oct 3;35(5):537-547. doi: 10.13075/ijomeh.1896.01942. Epub 2022 Jun 30. Int J Occup Med Environ Health. 2022. PMID: 35770786 Free PMC article.
-
Anti-SARS-CoV-2 Antibody Levels Associated With COVID-19 Protection in Outpatients Tested for SARS-CoV-2, US Flu Vaccine Effectiveness Network, October 2021-June 2022.J Infect Dis. 2024 Jul 25;230(1):45-54. doi: 10.1093/infdis/jiae090. J Infect Dis. 2024. PMID: 39052724 Free PMC article.
-
A bead-based multiplex assay covering all coronaviruses pathogenic for humans for sensitive and specific surveillance of SARS-CoV-2 humoral immunity.Sci Rep. 2023 Dec 9;13(1):21846. doi: 10.1038/s41598-023-48581-9. Sci Rep. 2023. PMID: 38071261 Free PMC article.
-
Multiplex Immunoassay Approaches Using Luminex® xMAP® Technology for the Study of COVID-19 Disease.Adv Exp Med Biol. 2023;1412:479-489. doi: 10.1007/978-3-031-28012-2_26. Adv Exp Med Biol. 2023. PMID: 37378784
References
-
- Rowe AD, Stoway SD, Ahlman H, Arora V, Caggana M, Fornari A, et al.. A Novel Approach to Improve Newborn Screening for Congenital Hypothyroidism by Integrating Covariate-Adjusted Results of Different Tests into CLIR Customized Interpretive Tools. Int J Neonatal Screen. 2021;7(2). 10.3390/ijns7020023 - DOI - PMC - PubMed
-
- Guthrie R, Susi A. A Simple Phenylalanine Method for Detecting Phenylketonuria in Large Populations of Newborn Infants. Pediatrics. 1963;32:338–43. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous

