PfSPZ-CVac efficacy against malaria increases from 0% to 75% when administered in the absence of erythrocyte stage parasitemia: A randomized, placebo-controlled trial with controlled human malaria infection
- PMID: 34048504
- PMCID: PMC8191919
- DOI: 10.1371/journal.ppat.1009594
PfSPZ-CVac efficacy against malaria increases from 0% to 75% when administered in the absence of erythrocyte stage parasitemia: A randomized, placebo-controlled trial with controlled human malaria infection
Abstract
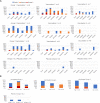
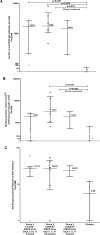
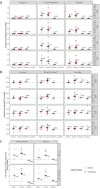
PfSPZ-CVac combines 'PfSPZ Challenge', which consists of infectious Plasmodium falciparum sporozoites (PfSPZ), with concurrent antimalarial chemoprophylaxis. In a previously-published PfSPZ-CVac study, three doses of 5.12x104 PfSPZ-CVac given 28 days apart had 100% vaccine efficacy (VE) against controlled human malaria infection (CHMI) 10 weeks after the last immunization, while the same dose given as three injections five days apart had 63% VE. Here, we conducted a dose escalation trial of similarly condensed schedules. Of the groups proceeding to CHMI, the first study group received three direct venous inoculations (DVIs) of a dose of 5.12x104 PfSPZ-CVac seven days apart and the next full dose group received three DVIs of a higher dose of 1.024x105 PfSPZ-CVac five days apart. CHMI (3.2x103 PfSPZ Challenge) was performed by DVI 10 weeks after the last vaccination. In both CHMI groups, transient parasitemia occurred starting seven days after each vaccination. For the seven-day interval group, the second and third vaccinations were therefore administered coincident with parasitemia from the prior vaccination. Parasitemia was associated with systemic symptoms which were severe in 25% of subjects. VE in the seven-day group was 0% (7/7 infected) and in the higher-dose, five-day group was 75% (2/8 infected). Thus, the same dose of PfSPZ-CVac previously associated with 63% VE when given on a five-day schedule in the prior study had zero VE here when given on a seven-day schedule, while a double dose given on a five-day schedule here achieved 75% VE. The relative contributions of the five-day schedule and/or the higher dose to improved VE warrant further investigation. It is notable that administration of PfSPZ-CVac on a schedule where vaccine administration coincided with blood-stage parasitemia was associated with an absence of sterile protective immunity. Clinical trials registration: NCT02773979.
Conflict of interest statement
I have read the journal’s policy and the authors of this manuscript have the following competing interests: SCM, SG, JKK, KWC, JGK and LAJ report no relevant conflicts of interest. GAD is employed by NIH (trial sponsor). BKLS, YA, NKC, SC, ERJ, SLH, and TLR are full time employees of Sanaria Inc. which is developing vaccines against malaria based on whole sporozoites, including PfSPZ-CVac and PfSPZ Vaccine. There are no patent applications or issued patents pertaining to the results presented herein.
Figures


Similar articles
-
Immunogenicity and Protective Efficacy of Radiation-Attenuated and Chemo-Attenuated PfSPZ Vaccines in Equatoguinean Adults.Am J Trop Med Hyg. 2021 Jan;104(1):283-293. doi: 10.4269/ajtmh.20-0435. Am J Trop Med Hyg. 2021. PMID: 33205741 Free PMC article. Clinical Trial.
-
Safety and efficacy of PfSPZ Vaccine against malaria in healthy adults and women anticipating pregnancy in Mali: two randomised, double-blind, placebo-controlled, phase 1 and 2 trials.Lancet Infect Dis. 2024 Dec;24(12):1366-1382. doi: 10.1016/S1473-3099(24)00360-8. Epub 2024 Aug 14. Lancet Infect Dis. 2024. PMID: 39153490 Clinical Trial.
-
Safety, Immunogenicity, and Protective Efficacy against Controlled Human Malaria Infection of Plasmodium falciparum Sporozoite Vaccine in Tanzanian Adults.Am J Trop Med Hyg. 2018 Aug;99(2):338-349. doi: 10.4269/ajtmh.17-1014. Epub 2018 Jun 21. Am J Trop Med Hyg. 2018. PMID: 29943719 Free PMC article. Clinical Trial.
-
Protective efficacy and safety of radiation-attenuated and chemo-attenuated Plasmodium Falciparum sporozoite vaccines against controlled and natural malaria infection: a systematic review and meta-analysis of randomized controlled trials.Infection. 2024 Jun;52(3):707-722. doi: 10.1007/s15010-024-02174-4. Epub 2024 Feb 6. Infection. 2024. PMID: 38319556
-
Progress with Plasmodium falciparum sporozoite (PfSPZ)-based malaria vaccines.Vaccine. 2015 Dec 22;33(52):7452-61. doi: 10.1016/j.vaccine.2015.09.096. Epub 2015 Nov 27. Vaccine. 2015. PMID: 26469720 Free PMC article. Review.
Cited by
-
Plasmodium falciparum infection coinciding with the malaria vaccine candidate BK-SE36 administration interferes with the immune responses in Burkinabe children.Front Immunol. 2023 Mar 10;14:1119820. doi: 10.3389/fimmu.2023.1119820. eCollection 2023. Front Immunol. 2023. PMID: 36993981 Free PMC article.
-
Malaria: Factors affecting disease severity, immune evasion mechanisms, and reversal of immune inhibition to enhance vaccine efficacy.PLoS Pathog. 2025 Jan 23;21(1):e1012853. doi: 10.1371/journal.ppat.1012853. eCollection 2025 Jan. PLoS Pathog. 2025. PMID: 39847577 Free PMC article. Review.
-
Sterile protection against relapsing malaria with a single-shot vaccine.NPJ Vaccines. 2022 Oct 27;7(1):126. doi: 10.1038/s41541-022-00555-0. NPJ Vaccines. 2022. PMID: 36302860 Free PMC article.
-
Malaria blood stage infection suppresses liver stage infection via host-induced interferons but not hepcidin.Nat Commun. 2024 Mar 7;15(1):2104. doi: 10.1038/s41467-024-46270-3. Nat Commun. 2024. PMID: 38453916 Free PMC article.
-
A PfSPZ vaccine immunization regimen equally protective against homologous and heterologous controlled human malaria infection.NPJ Vaccines. 2022 Aug 23;7(1):100. doi: 10.1038/s41541-022-00510-z. NPJ Vaccines. 2022. PMID: 35999221 Free PMC article.
References
Publication types
MeSH terms
Substances
Associated data
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous

