Camidanlumab tesirine in patients with relapsed or refractory lymphoma: a phase 1, open-label, multicentre, dose-escalation, dose-expansion study
- PMID: 34048682
- PMCID: PMC9241579
- DOI: 10.1016/S2352-3026(21)00103-4
Camidanlumab tesirine in patients with relapsed or refractory lymphoma: a phase 1, open-label, multicentre, dose-escalation, dose-expansion study
Abstract
Background: Novel approaches are required to improve outcomes in relapsed or refractory classical Hodgkin lymphoma and non-Hodgkin lymphoma. We aimed to evaluate camidanlumab tesirine, an anti-CD25 antibody-drug conjugate, in this patient population.
Methods: This was a phase 1, dose-escalation (part 1), dose-expansion (part 2), multicentre trial done in 12 hospital sites (seven in the USA and five in the UK). Adults (≥18 years old) with pathologically confirmed relapsed or refractory classical Hodgkin lymphoma or non-Hodgkin lymphoma, an Eastern Cooperative Oncology Group performance status 0-2, who had no therapies available to them with established clinical benefit for their disease stage were enrolled. Camidanlumab tesirine was administered intravenously (3-150 μg/kg) once every 3 weeks. Primary objectives were to assess dose-limiting toxicity, determine maximum tolerated dose and recommended expansion dose(s), and assess safety of camidanlumab tesirine. Safety was assessed in all treated patients; antitumour activity was assessed in patients with one or more valid baseline and post-baseline disease assessment and in those who had disease progression or died after first study-drug dose. This trial was registered with ClinicalTrials.gov, NCT02432235.
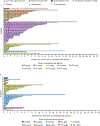
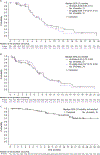
Findings: Between Oct 5, 2015, and Jun 30, 2019, 133 patients were enrolled (77 [58%] had classical Hodgkin lymphoma and 56 (42%) had non-Hodgkin lymphoma). Median follow-up was 9·2 months (IQR 4·2-14·3). Eight dose-limiting toxicities were reported in five (6%) of 86 patients who were evaluable; the maximum tolerated dose was not reached. The recommended doses for expansion were 30 μg/kg and 45 μg/kg for patients with classical Hodgkin lymphoma and 80 μg/kg for patients with T-cell non-Hodgkin lymphomas. No recommended doses for expansion were defined for B-cell non-Hodgkin lymphomas. Grade 3 or worse treatment-emergent adverse events (reported by ≥10% of the 133 patients) included increased γ-glutamyltransferase (20 [15%] patients), maculopapular rash (16 [12%]), and anaemia (15 [11%]); 74 (56%) patients had serious treatment-emergent adverse events, most commonly pyrexia (16 [12%]). One (1%) fatal treatment-emergent adverse event and two (2%) deaths outside the reporting period were considered at least possibly study-drug related. Antitumoural activity was seen in classical Hodgkin and non-Hodgkin lymphomas; notably in all patients with classical Hodgkin lymphoma, the overall response was 71% (95% CI 60-81).
Interpretation: These results warrant evaluation of camidanlumab tesirine as a potential treatment option for relapsed or refractory lymphoma, particularly in patients with classical Hodgkin lymphoma.
Funding: ADC Therapeutics.
Copyright © 2021 Elsevier Ltd. All rights reserved.
Conflict of interest statement
Declaration of interests MH reports grants from Takeda, Spectrum Pharmaceuticals, Astellas Pharma; and personal fees from Janssen, Incyte Corporation, ADC Therapeutics, Celgene Corporation, Pharmacyclics, Omeros, AbGenomics, Verastem, TeneoBio, Sanofi Genzyme, BeiGene, and AstraZeneca, outside the submitted work. GPC reports personal fees from ADC Therapeutics, during the study; personal fees from Roche, Takeda, Bristol-Myers Squibb, Merck Sharp & Dohme, Celleron, BeiGene, Gilead, Novartis, Celgene, and Amgen, outside the submitted work. PFC reports grants from ADC Therapeutics, during the the study; grants from Genentech, outside the submitted work; and personal fees from ADC Therapeutics, Kite Therapeutics, Amgen, Bayer, Verastem, Seattle Genetics, TG Therapeutics, and Celgene, outside the submitted work. FS reports personal fees from Imbrium Therapeutics, outside the submitted work. AS reports personal fees from ADC Therapeutics, during the study. AD reports grants from ADC Therapeutics; grants, personal fees and non-financial support from Roche/Genentech, Celgene; grants and personal fees from Gilead/Kite, Accerta Pharma/AstraZeneca, Karyopharma; and personal fees from Janssen, MorphoSys, and Takeda, during the conduct of the study. JR reports personal fees from Takeda, ADC Therapeutics, Bristol-Myers Squibb, Novartis, Kite Pharma, Seattle Genetics; and reports his spouse holds stocks in AstraZeneca, GlaxoSmithKline, and ADC Therapeutics, outside of the submitted work. TM reports personal fees and non-financial support from Amgen; non-financial support from Jazz, Pfizer, Bayer, Kyowa Kirin, and Celgene; personal fees and non-financial support from Gilead/Kite; and personal fees from Novartis, Daiichi Sankyo, Atara, Takeda, Janssen, Roche, and AstraZeneca, outside the submitted work. JMZ reports personal fees from Seattle Genetics, Kyowa Kirin, and Verastem, outside the submitted work. PF reports personal fees from ADC Therapeutics, Takeda, Roche, and Merck, outside the submitted work. KH reports personal fees from ADC Therapeutics, during the study. HGC reports personal fees from, employment with, and stocks in ADC Therapeutics, during the study. ShH reports personal fees from ADC Therapeutics, during the study. JB reports personal fees from, employment with, and stocks in ADC Therapeutics, during the study. JF reports personal fees from ADC Therapeutics, during the study. JW reports personal fees from, employment with, and stocks in ADC Therapeutics, during the study. StH reports grants and personal fees from ADC Therapeutics, Celgene, Kyowa Hakko Kirin, Seattle Genetics, Millennium/Takeda, Verastem and Secura Bio, Aileron, Forty Seven, Trillium Therapeutics, and Affimed; personal fees from C4 Therapeutics, Janssen, Kura Oncology, Myeloid Therapeutics, Vividion Therapeutics, Portola Pharmaceuticals, Acrotech, Astex, BeiGene, Miragen, Merck, Innate Pharma, Bristol-Myers Squibb, Mundipharma, and ONO Pharma; and grants from Daiichi Sankyo, outside the submitted work. AK declares no competing interests.
Figures
Comment in
-
Anti-CD25 antibody-drug conjugates: improving the delivery of death to lymphoma cells?Lancet Haematol. 2021 Jun;8(6):e387-e389. doi: 10.1016/S2352-3026(21)00139-3. Lancet Haematol. 2021. PMID: 34048676 No abstract available.
Similar articles
-
Loncastuximab tesirine in relapsed or refractory diffuse large B-cell lymphoma (LOTIS-2): a multicentre, open-label, single-arm, phase 2 trial.Lancet Oncol. 2021 Jun;22(6):790-800. doi: 10.1016/S1470-2045(21)00139-X. Epub 2021 May 11. Lancet Oncol. 2021. PMID: 33989558 Clinical Trial.
-
Camidanlumab tesirine, an antibody-drug conjugate, in relapsed/refractory CD25-positive acute myeloid leukemia or acute lymphoblastic leukemia: A phase I study.Leuk Res. 2020 Aug;95:106385. doi: 10.1016/j.leukres.2020.106385. Epub 2020 Jun 7. Leuk Res. 2020. PMID: 32521310 Clinical Trial.
-
Avadomide plus obinutuzumab in patients with relapsed or refractory B-cell non-Hodgkin lymphoma (CC-122-NHL-001): a multicentre, dose escalation and expansion phase 1 study.Lancet Haematol. 2020 Sep;7(9):e649-e659. doi: 10.1016/S2352-3026(20)30208-8. Epub 2020 Aug 3. Lancet Haematol. 2020. PMID: 32758434 Clinical Trial.
-
CD25-targeted antibody-drug conjugate camidanlumab tesirine for relapsed or refractory classical Hodgkin lymphoma.Invest New Drugs. 2022 Dec;40(6):1333-1341. doi: 10.1007/s10637-022-01300-z. Epub 2022 Sep 8. Invest New Drugs. 2022. PMID: 36074313 Review.
-
A New Target for Hodgkin Lymphoma - Camidanlumab Tesirine.Curr Hematol Malig Rep. 2021 Feb;16(1):19-24. doi: 10.1007/s11899-021-00604-w. Epub 2021 Jan 25. Curr Hematol Malig Rep. 2021. PMID: 33492560 Review.
Cited by
-
New molecular targets in Hodgkin and Reed-Sternberg cells.Front Immunol. 2023 May 15;14:1155468. doi: 10.3389/fimmu.2023.1155468. eCollection 2023. Front Immunol. 2023. PMID: 37266436 Free PMC article. Review.
-
A B7-H4-Targeting Antibody-Drug Conjugate Shows Antitumor Activity in PARPi and Platinum-Resistant Cancers with B7-H4 Expression.Clin Cancer Res. 2024 Apr 15;30(8):1567-1581. doi: 10.1158/1078-0432.CCR-23-1079. Clin Cancer Res. 2024. PMID: 37882675 Free PMC article.
-
PTCL, NOS: An update on classification, risk-stratification, and treatment.Front Oncol. 2023 Feb 9;13:1101441. doi: 10.3389/fonc.2023.1101441. eCollection 2023. Front Oncol. 2023. PMID: 36845711 Free PMC article. Review.
-
Barriers to achieving a cure in lymphoma.Cancer Drug Resist. 2021 Nov 5;4(4):965-983. doi: 10.20517/cdr.2021.66. eCollection 2021. Cancer Drug Resist. 2021. PMID: 35582375 Free PMC article. Review.
-
Redefining Precision Management of r/r Large B-Cell Lymphoma: Novel Antibodies Take on CART and BMT in the Quest for Future Treatment Strategies.Cells. 2023 Jul 14;12(14):1858. doi: 10.3390/cells12141858. Cells. 2023. PMID: 37508523 Free PMC article. Review.
References
-
- Armitage JO, Gascoyne RD, Lunning MA, Cavalli F. Non-Hodgkin lymphoma. Lancet 2017; 390(10091): 298–310. - PubMed
-
- Eichenauer DA, Aleman BMP, Andre M, et al. Hodgkin lymphoma: ESMO Clinical Practice Guidelines for diagnosis, treatment and follow-up. Ann Oncol 2018; 29(Suppl 4): iv19–iv29. - PubMed
-
- Ansell SM. Hodgkin lymphoma: 2016 update on diagnosis, risk-stratification, and management. Am J Hematol 2016; 91(4): 434–42. - PubMed
-
- Moskowitz CH, Nademanee A, Masszi T, et al. Brentuximab vedotin as consolidation therapy after autologous stem-cell transplantation in patients with Hodgkin’s lymphoma at risk of relapse or progression (AETHERA): a randomised, double-blind, placebo-controlled, phase 3 trial. Lancet 2015; 385(9980): 1853–62. - PubMed
Publication types
MeSH terms
Substances
Associated data
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous

