Coupling the in vivo performance to the in vitro characterization of PLGA microparticles
- PMID: 34048931
- PMCID: PMC9435463
- DOI: 10.1016/j.ijpharm.2021.120738
Coupling the in vivo performance to the in vitro characterization of PLGA microparticles
Abstract
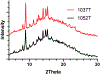
The main objective of the study was to determine if rodent housing conditions, specifically housing climate, could impact the in vivo performance of poly(lactide-co-glycolide) (PLGA) microspheres through temperature modification of the subcutaneous space. Vivitrol®, a once monthly naltrexone injectable suspension, was chosen as a model PLGA microparticle formulation for this study. Two lots of Vivitrol were used to ascertain any potential differences that may exist between the batches and if in vitro characterization techniques could delineate any variation(s). The pharmacokinetics of the naltrexone-PLGA microparticles were determined in the rodent model under two different housing climates (20 vs. 25 °C). The results demonstrate that such difference in housing temperature resulted in a change in subcutaneous temperature but actually within a narrow range (36.31-36.77 °C) and thus minimally influenced the in vivo performance of subcutaneously injected microparticles. The shake-flask method was used to characterize the in vitro release at 35, 36, and 37 °C and demonstrated significant differences in the in vitro release profiles across this range of temperatures. Minimal differences in the in vitro characterization of the two lots were found. While these results did not provide statistical significance, the local in vivo temperature may be a parameter that should be considered when evaluating microparticle performance. The IVIVCs demonstrate that in vitro release at 37 °C may not accurately represent the in vivo conditions (i.e., subcutaneous space in rodents), and in certain instances lower in vitro release temperatures may more accurately represent the in vivo microenvironment and provide better correlations. Future studies will determine the extent temperature and specifically co-housing, may have on the relative impact of the in vivo performance of injectable polymeric microparticles based upon the significant differences observed in the in vitro release profiles across the range of 35-37 °C.
Keywords: In vivo-in vitro correlation; Naltrexone; PLGA microparticles; Pharmacokinetics; Vivitrol.
Copyright © 2021. Published by Elsevier B.V.
Figures
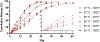
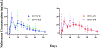



Similar articles
-
In vitro-in vivo correlation of PLGA microspheres: Effect of polymer source variation and temperature.J Control Release. 2022 Jul;347:347-355. doi: 10.1016/j.jconrel.2022.05.014. Epub 2022 May 18. J Control Release. 2022. PMID: 35569590
-
The Impact of Post-Processing Temperature on PLGA Microparticle Properties.Pharm Res. 2023 Nov;40(11):2677-2685. doi: 10.1007/s11095-023-03568-z. Epub 2023 Aug 17. Pharm Res. 2023. PMID: 37589826 Free PMC article.
-
Continuous in-line homogenization process for scale-up production of naltrexone-loaded PLGA microparticles.J Control Release. 2020 Sep 10;325:347-358. doi: 10.1016/j.jconrel.2020.06.023. Epub 2020 Jul 7. J Control Release. 2020. PMID: 32645336 Free PMC article.
-
Formulation composition, manufacturing process, and characterization of poly(lactide-co-glycolide) microparticles.J Control Release. 2021 Jan 10;329:1150-1161. doi: 10.1016/j.jconrel.2020.10.044. Epub 2020 Oct 24. J Control Release. 2021. PMID: 33148404 Free PMC article. Review.
-
Long-acting PLGA microspheres: Advances in excipient and product analysis toward improved product understanding.Adv Drug Deliv Rev. 2023 Jul;198:114857. doi: 10.1016/j.addr.2023.114857. Epub 2023 May 5. Adv Drug Deliv Rev. 2023. PMID: 37149041 Review.
Cited by
-
Biodegradable Long-Acting Injectables: Platform Technology and Industrial Challenges.Handb Exp Pharmacol. 2024;284:133-150. doi: 10.1007/164_2023_651. Handb Exp Pharmacol. 2024. PMID: 37059910
-
PLGA-based long-acting injectable (LAI) formulations.J Control Release. 2025 Jun 10;382:113758. doi: 10.1016/j.jconrel.2025.113758. Epub 2025 Apr 21. J Control Release. 2025. PMID: 40268201 Review.
-
Advanced Electrospun Chitosan-(Polylactic Acid)-(Silver Nanoparticle)-Based Scaffolds for Facilitated Healing of Purulent Wounds: A Preclinical Investigation.Polymers (Basel). 2025 Aug 15;17(16):2225. doi: 10.3390/polym17162225. Polymers (Basel). 2025. PMID: 40871172 Free PMC article.
-
Long-Acting injectable buprenorphine PLGA microparticle formulation.Int J Pharm. 2025 Oct 15;683:126006. doi: 10.1016/j.ijpharm.2025.126006. Epub 2025 Jul 27. Int J Pharm. 2025. PMID: 40730319
-
Scanning Analysis of Sequential Semisolvent Vapor Impact To Study Naltrexone Release from Poly(lactide-co-glycolide) Microparticles.Mol Pharm. 2022 Nov 7;19(11):4286-4298. doi: 10.1021/acs.molpharmaceut.2c00595. Epub 2022 Sep 27. Mol Pharm. 2022. PMID: 36166409 Free PMC article.
References
-
- 2006. Clinical Pharmacology and Biopharmaceutics Review. Application Number 21-897; February/2006.
-
- Acharya G, Shin CS, Vedantham K, McDermott M, Rish T, Hansen K, Fu Y, Park K, 2010. A study of drug release from homogeneous PLGA microstructures. J. Control. Release 146, 201–206. - PubMed
-
- Allison SD, 2008. Effect Of Structural Relaxation On The Preparation And Drug Release Behavior Of Poly(lactic-co-glycolic)acid Microparticle Drug Delivery Systems. J. Pharm. Sci 97, 2022–2035. - PubMed
-
- Amann LC, Gandal MJ, Lin R, Liang Y, Siegel SJ, 2010. In vitro-in vivo correlations of scalable PLGA-risperidone implants for the treatment of schizophrenia. Pharm Res 27, 1730–1737. - PubMed
-
- Andhariya JV, Choi S, Wang Y, Zou Y, Burgess DJ, Shen J, 2017a. Accelerated in vitro release testing method for naltrexone loaded PLGA microspheres. Int. J. Pharm 520, 79–85. - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

