Assessment of cognitive and psychomotor impairment, subjective effects, and blood THC concentrations following acute administration of oral and vaporized cannabis
- PMID: 34049452
- PMCID: PMC9361180
- DOI: 10.1177/02698811211021583
Assessment of cognitive and psychomotor impairment, subjective effects, and blood THC concentrations following acute administration of oral and vaporized cannabis
Abstract
Background: Cannabis legalization is expanding, but there are no established methods for detecting cannabis impairment.
Aim: Characterize the acute impairing effects of oral and vaporized cannabis using various performance tests.
Methods: Participants (N = 20, 10 men/10 women) who were infrequent cannabis users ingested cannabis brownies (0, 10, and 25 mg Δ-9-tetrahydrocannabinol, THC) and inhaled vaporized cannabis (0, 5, and 20 mg THC) in six double-blind outpatient sessions. Cognitive/psychomotor impairment was assessed with a battery of computerized tasks sensitive to cannabis effects, a novel test (the DRiving Under the Influence of Drugs, DRUID®), and field sobriety tests. Blood THC concentrations and subjective drug effects were evaluated.
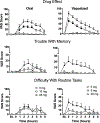
Results: Low oral/vaporized doses did not impair cognitive/psychomotor performance relative to placebo but produced positive subjective effects. High oral/vaporized doses impaired cognitive/psychomotor performance and increased positive and negative subjective effects. The DRUID® was the most sensitive test to cannabis impairment, as it detected significant differences between placebo and active doses within both routes of administration. Women displayed more impairment on the DRUID® than men at the high vaporized dose only. Field sobriety tests showed little sensitivity to cannabis-induced impairment. Blood THC concentrations were far lower after cannabis ingestion versus inhalation. After inhalation, blood THC concentrations typically returned to baseline well before pharmacodynamic effects subsided.
Conclusions: Standard approaches for identifying impairment due to cannabis exposure (i.e. blood THC and field sobriety tests) have severe limitations. There is a need to identify novel biomarkers of cannabis exposure and/or behavioral tests like the DRUID® that can reliably and accurately detect cannabis impairment at the roadside and in the workplace.
Keywords: Cannabis; cannabis edibles; cannabis vaporizers; impairment.
Conflict of interest statement
Declaration of conflicting interests
The author(s) declared the following potential conflicts of interest with respect to the research, authorship, and/or publication of this article: Dr. Spindle has served as a consultant for Canopy Health Innovations Inc. Dr. Vandrey has served as a consultant or received honoraria from Canopy Health Innovations Inc., FSD Pharma, and Present Life Corporation. Michael Milburn is the creator of the DRUID® application and is the Chief Scientific Officer (CSO) and a stockholder of Impairment Science, Inc. The remaining authors have no conflicts of interest to declare.
Figures




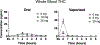
References
-
- Abrams DI, Vizoso HP, Shade SB, et al. (2007) Vaporization as a smokeless cannabis delivery system: A pilot study. Clin Pharmacol Ther 82: 572–578. - PubMed
-
- Bosker WM, Kuypers KP, Theunissen EL, et al. (2012) Medicinal Delta(9)-tetrahydrocannabinol (dronabinol) impairs on-the-road driving performance of occasional and heavy cannabis users but is not detected in Standard Field Sobriety Tests. Addiction 107: 1837–1844. - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

