Intra-species sialic acid polymorphism in humans: a common niche for influenza and coronavirus pandemics?
- PMID: 34049471
- PMCID: PMC8208123
- DOI: 10.1080/22221751.2021.1935329
Intra-species sialic acid polymorphism in humans: a common niche for influenza and coronavirus pandemics?
Abstract
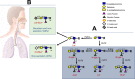
The ongoing COVID-19 pandemic has led to more than 159 million confirmed cases with over 3.3 million deaths worldwide, but it remains mystery why most infected individuals (∼98%) were asymptomatic or only experienced mild illness. The same mystery applies to the deadly 1918 H1N1 influenza pandemic, which has puzzled the field for a century. Here we discuss dual potential properties of the 1918 H1N1 pandemic viruses that led to the high fatality rate in the small portion of severe cases, while about 98% infected persons in the United States were self-limited with mild symptoms, or even asymptomatic. These variations now have been postulated to be impacted by polymorphisms of the sialic acid receptors in the general population. Since coronaviruses (CoVs) also recognize sialic acid receptors and cause severe acute respiratory syndrome epidemics and pandemics, similar principles of influenza virus evolution and pandemicity may also apply to CoVs. A potential common principle of pathogen/host co-evolution of influenza and CoVs under selection of host sialic acids in parallel with different epidemic and pandemic influenza and coronaviruses is discussed.
Keywords: 1918 H1N1 pandemic; Influenza and coronavirus; co-evolution; polymorphism; sialic acid receptor.
Conflict of interest statement
No potential conflict of interest was reported by the author(s).
Figures
Similar articles
-
Comparison of patients hospitalized with COVID-19, H7N9 and H1N1.Infect Dis Poverty. 2020 Dec 2;9(1):163. doi: 10.1186/s40249-020-00781-5. Infect Dis Poverty. 2020. PMID: 33261654 Free PMC article.
-
SARS-CoV-2 Evolutionary Adaptation toward Host Entry and Recognition of Receptor O-Acetyl Sialylation in Virus-Host Interaction.Int J Mol Sci. 2020 Jun 26;21(12):4549. doi: 10.3390/ijms21124549. Int J Mol Sci. 2020. PMID: 32604730 Free PMC article. Review.
-
Enhanced Human-Type Receptor Binding by Ferret-Transmissible H5N1 with a K193T Mutation.J Virol. 2018 Apr 27;92(10):e02016-17. doi: 10.1128/JVI.02016-17. Print 2018 May 15. J Virol. 2018. PMID: 29491160 Free PMC article.
-
Tropism and Infectivity of a Seasonal A(H1N1) and a Highly Pathogenic Avian A(H5N1) Influenza Virus in Primary Differentiated Ferret Nasal Epithelial Cell Cultures.J Virol. 2019 May 1;93(10):e00080-19. doi: 10.1128/JVI.00080-19. Print 2019 May 15. J Virol. 2019. PMID: 30814288 Free PMC article.
-
The Pandemic Threat of Emerging H5 and H7 Avian Influenza Viruses.Viruses. 2018 Aug 28;10(9):461. doi: 10.3390/v10090461. Viruses. 2018. PMID: 30154345 Free PMC article. Review.
Cited by
-
Epipharyngeal Abrasive Therapy Down-regulates the Expression of Cav1.2: A Key Molecule in Influenza Virus Entry.In Vivo. 2022 Sep-Oct;36(5):2357-2364. doi: 10.21873/invivo.12967. In Vivo. 2022. PMID: 36099101 Free PMC article.
-
Have Diagnostics, Therapies, and Vaccines Made the Difference in the Pandemic Evolution of COVID-19 in Comparison with "Spanish Flu"?Pathogens. 2023 Jun 23;12(7):868. doi: 10.3390/pathogens12070868. Pathogens. 2023. PMID: 37513715 Free PMC article. Review.
-
PEDV: Insights and Advances into Types, Function, Structure, and Receptor Recognition.Viruses. 2022 Aug 9;14(8):1744. doi: 10.3390/v14081744. Viruses. 2022. PMID: 36016366 Free PMC article. Review.
-
Comprehensive N-glycosylation profiling of recombinant spike S1 protein from the wild-type SARS-CoV-2 and its variants.Front Immunol. 2025 Jul 16;16:1592142. doi: 10.3389/fimmu.2025.1592142. eCollection 2025. Front Immunol. 2025. PMID: 40755778 Free PMC article.
References
-
- Morens DM, Fauci AS.. The 1918 influenza pandemic: insights for the 21st century. J Infect Dis. 2007;195(7):1018–1028. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
