The association between obesity and weight loss after bariatric surgery on the vaginal microbiota
- PMID: 34049596
- PMCID: PMC8164250
- DOI: 10.1186/s40168-021-01011-2
The association between obesity and weight loss after bariatric surgery on the vaginal microbiota
Abstract
Background: Obesity and vaginal microbiome (VMB) dysbiosis are each risk factors for adverse reproductive and oncological health outcomes in women. Here, we investigated the relationship between obesity, vaginal bacterial composition, local inflammation and bariatric surgery.
Methods: Vaginal bacterial composition assessed by high-throughput sequencing of bacterial 16S rRNA genes and local cytokine levels measured using a multiplexed Magnetic Luminex Screening Assay were compared between 67 obese and 42 non-obese women. We further assessed temporal changes in the microbiota and cytokines in a subset of 27 women who underwent bariatric surgery.
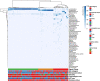
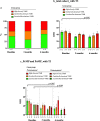
Results: The bacterial component of the vaginal microbiota in obese women was characterised by a lower prevalence of a Lactobacillus-dominant VMB and higher prevalence of a high diversity (Lactobacillus spp., and Gardnerella- spp. depleted) VMB, compared with non-obese subjects (p<0.001). Obese women had higher relative abundance of Dialister species (p<0.001), Anaerococcus vaginalis (p=0.021), and Prevotella timonensis (p=0.020) and decreased relative abundance of Lactobacillus crispatus (p=0.014). Local vaginal IL-1β, IL-4, IL-6, IL-8, IFNγ, MIP-1α and TNFα levels were all higher among obese women, however, only IL-1β and IL-8 correlated with VMB species diversity. In a subset of obese women undergoing bariatric surgery, there were no significant overall differences in VMB following surgery; however, 75% of these women remained obese at 6 months. Prior to surgery, there was no relationship between body mass index (BMI) and VMB structure; however, post-surgery women with a Lactobacillus-dominant VMB had a significantly lower BMI than those with a high diversity VMB.
Conclusions: Obese women have a significantly different vaginal microbiota composition with increased levels of local inflammation compared to non-obese women. Bariatric surgery does not change the VMB; however, those with the greatest weight loss 6-month post-surgery are most likely to have a Lactobacillus-dominant VMB. Video abstract.
Keywords: BMI; Bariatric surgery; Body mass index; Obesity; Overweight; Vaginal microbiota.
Conflict of interest statement
The authors declare no competing interests.
Figures


References
Publication types
MeSH terms
Substances
Supplementary concepts
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous

