Pretomanid with bedaquiline and linezolid for drug-resistant TB: a comparison of prospective cohorts
- PMID: 34049607
- PMCID: PMC8171246
- DOI: 10.5588/ijtld.21.0035
Pretomanid with bedaquiline and linezolid for drug-resistant TB: a comparison of prospective cohorts
Abstract
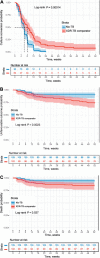
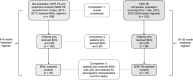
BACKGROUND: There are no data comparing the 6-9 month oral three-drug Nix regimen (bedaquiline, pretomanid and linezolid [BPaL]) to conventional regimens containing bedaquiline (B, BDQ) and linezolid (L, LZD).METHODS: Six-month post end-of-treatment outcomes were compared between Nix-TB (n = 109) and 102 prospectively recruited extensively drug-resistant TB patients who received an ˜18-month BDQ-based regimen (median of 8 drugs). A subset of patients received BDQ and LZD (n = 86), and a subgroup of these (n = 75) served as individually matched controls in a pairwise comparison to determine differences in regimen efficacy.RESULTS: Favourable outcomes (%) were significantly better with BPaL than with the B-L-based combination regimen (98/109, 89.9% vs. 56/86, 65.1%; adjusted relative risk ratio [aRRR] 1.35; P < 0.001) and in the matched pairwise analysis (67/75, 89.3% vs. 48/75, 64.0%; aRRR 1.39; P = 0.001), despite significantly higher baseline bacterial load and prior second-line drug exposure in the BPaL cohort. Time to culture conversion (P < 0.001), time to unfavourable outcome (P < 0.01) and time to death (P < 0.03) were significantly better or lower with BPaL than the B-L-based combinations.CONCLUSION: The BPaL regimen (and hence substitution of multiple other drugs by pretomanid and/or higher starting-dose LZD) may improve outcomes in drug-resistant TB patients with poor prognostic features. However, prospective controlled studies are required to definitively answer this question.
CONTEXTE :: Il n’y a pas de données comparant les protocoles oraux de 6–9 mois associant trois médicaments (bédaquiline, prétomanide et linézolide [BPaL]) aux protocoles conventionnels contenant de la bédaquiline (B, BDQ) et du linézolide (L, LZD).
MÉTHODE :: On a comparé les résultats 6 mois après la fin du traitement entre Nix-TB (n = 109) et 102 patients TB recrutés prospectivement qui ont reçu un protocole de BDQ d’environ 18 mois (médiane de 8 médicaments). Un sous ensemble de patients a reçu de la BDQ et du LZD (n = 86) et un sous-groupe de ces derniers (n = 75) a servi de témoins appariés individuellement pour des comparaisons par paires afin de déterminer les différences d’efficacité du protocole.
RÉSULTATS :: Des résultats favorables (%) ont été significativement meilleurs avec BPaL qu’avec le protocole combiné basé sur B-L (98/109, 89,9% contre 56/86, 65,1% ; rapport de risque relatif ajusté [RRRa] 1,35 ; P < 0,001) et dans l’analyse par paires (67/75, 89,3% contre 48/75, 64,0% ; RRRa 1,39 ; P = 0,001) malgré une charge bactérienne initiale significativement plus élevée et une exposition préalable aux médicaments de deuxième ligne. Le délai de conversion de la culture (P < 0,001), le délai de résultats défavorables (P < 0,01) et de décès (P < 0,03) ont été significativement meilleurs ou plus bas avec BPaL comparés à la combinaison à base de BL.
CONCLUSION :: Le protocole BPaL (et donc la substitution de multiples autres médicaments par le prétomanide et/une dose de départ plus élevée de LZD) pourrait améliorer les résultats des patients TB résistante ayant des facteurs de pronostic négatifs. Des études prospectives contrôlées sont cependant requises pour répondre définitivement à cette question.
Figures
Comment in
-
Shortening MDR-TB treatment: is treating more patients with fewer drugs better?Int J Tuberc Lung Dis. 2021 Jun 1;25(6):419-420. doi: 10.5588/ijtld.21.0146. Int J Tuberc Lung Dis. 2021. PMID: 34049601 No abstract available.
References
-
- Dheda K, et al. Outcomes, infectiousness, and transmission dynamics of patients with extensively drug-resistant tuberculosis and home-discharged patients with programmatically incurable tuberculosis: a prospective cohort study. Lancet Respir Med. 2017;5(4):269–281. - PubMed
-
- Dheda K, et al. The Lancet Respiratory Medicine Commission: 2019 update: epidemiology, pathogenesis, transmission, diagnosis, and management of multidrug-resistant and incurable tuberculosis. Lancet Respir Med. 2019;7(9):820–826. - PubMed
-
- World Health Organization Geneva, Switzerland: WHO; 2019. Global tuberculosis report, 2019.
-
- Olayanju O, et al. Long-term bedaquiline-related treatment outcomes in patients with extensively drug-resistant tuberculosis from South Africa. Eur Respir J. 2018;51(5):1800544. - PubMed
-
- Ndjeka N, et al. High treatment success rate for multidrug-resistant and extensively drug-resistant tuberculosis using a bedaquiline-containing treatment regimen. Eur Respir J. 2018;52(6):1801528. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

