Two ligand-binding sites in CO-reducing V nitrogenase reveal a general mechanistic principle
- PMID: 34049880
- PMCID: PMC8163085
- DOI: 10.1126/sciadv.abg4474
Two ligand-binding sites in CO-reducing V nitrogenase reveal a general mechanistic principle
Abstract
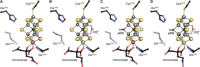
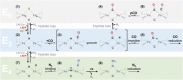
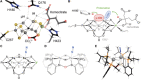
Besides its role in biological nitrogen fixation, vanadium-containing nitrogenase also reduces carbon monoxide (CO) to hydrocarbons, in analogy to the industrial Fischer-Tropsch process. The protein yields 93% of ethylene (C2H4), implying a C-C coupling step that mandates the simultaneous binding of two CO at the active site FeV cofactor. Spectroscopic data indicated multiple CO binding events, but structural analyses of Mo and V nitrogenase only confirmed a single site. Here, we report the structure of a two CO-bound state of V nitrogenase at 1.05 Å resolution, with one μ-bridging and one terminal CO molecule. This additional, specific ligand binding site suggests a mechanistic route for CO reduction and hydrocarbon formation, as well as a second access pathway for protons required during the reaction. Moreover, carbonyls are strong-field ligands that are chemically similar to mechanistically relevant hydrides that may be formed and used in a fully analogous fashion.
Copyright © 2021 The Authors, some rights reserved; exclusive licensee American Association for the Advancement of Science. No claim to original U.S. Government Works. Distributed under a Creative Commons Attribution NonCommercial License 4.0 (CC BY-NC).
Figures


References
-
- Burgess B. K., Lowe D. J., Mechanism of molybdenum nitrogenase. Chem. Rev. 96, 2983–3012 (1996). - PubMed
-
- Hu Y., Ribbe M. W., Historic overview of nitrogenase research. Methods Mol. Biol. 766, 3–7 (2011). - PubMed
-
- Einsle O., Nitrogenase FeMo cofactor: An atomic structure in three simple steps. J. Biol. Inorg. Chem. 19, 737–745 (2014). - PubMed
Publication types
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

