Experience of establishing and coordinating a nationwide network for bidirectional intussusception surveillance in India: lessons for multisite research studies
- PMID: 34049918
- PMCID: PMC8166592
- DOI: 10.1136/bmjopen-2020-046827
Experience of establishing and coordinating a nationwide network for bidirectional intussusception surveillance in India: lessons for multisite research studies
Abstract
Objectives: To document and share the process of establishing the nationally representative multisite surveillance network for intussusception in India, coordination, data management and lessons learnt from the implementation.
Design: This study combined both retrospective and prospective surveillance approaches.
Setting: 19 tertiary care institutions were selected in India considering the geographic representation and public and private mix PARTICIPANTS: All children under-2 years of age with intussusception PRIMARY AND SECONDARY OUTCOME MEASURES: The experience of site selection, regulatory approvals, data collection, quality assurance and network coordination were documented.
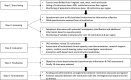
Results: The site selection process involved systematic and objective four steps including shortlisting of potential institutions, information seeking and telephonic interaction, site visits and site selection using objective criteria. Out of over 400 hospitals screened across India, 40 potential institutions were shortlisted and information was sought by questionnaire and interaction with investigators. Out of these, 25 institutes were visited and 19 sites were finally selected to participate in the study. The multistep selection process allowed filtering and identification of sites with adequate capacity and motivated investigators. The retrospective surveillance documented 1588 cases (range: 14-652 cases/site) and prospective surveillance recruited 621 cases (range: 5-191 cases/site). The multilayer quality assurance measures monitored and ensured protocol adherence, complete record retrieval and data completeness. The key challenges experienced included time taken for obtaining regulatory and ethical approvals, which delayed completion of the study. Ten sites continued with another multisite vaccine safety surveillance study.
Conclusion: The experience and results of this systematic and objective site selection method in India are promising. The systematic multistep site selection and data quality assurance methods presented here are feasible and practical. The lessons from the establishment and coordination of this surveillance network can be useful in planning, selecting the sites and conducting multisite and surveillance studies in India and developing countries.
Keywords: community child health; epidemiology; paediatric gastroenterology; public health.
© Author(s) (or their employer(s)) 2021. Re-use permitted under CC BY. Published by BMJ.
Conflict of interest statement
Competing interests: None declared.
Figures



References
-
- World Health Organisation . Post-marketing surveillance of rotavirus vaccine safety [Internet], 2017. Available: http://apps.who.int/iris/bitstream/10665/70017/1/WHO_IVB_09.01_eng.pdf
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Other Literature Sources
