In situ delivery of iPSC-derived dendritic cells with local radiotherapy generates systemic antitumor immunity and potentiates PD-L1 blockade in preclinical poorly immunogenic tumor models
- PMID: 34049930
- PMCID: PMC8166607
- DOI: 10.1136/jitc-2021-002432
In situ delivery of iPSC-derived dendritic cells with local radiotherapy generates systemic antitumor immunity and potentiates PD-L1 blockade in preclinical poorly immunogenic tumor models
Abstract
Background: Dendritic cells (DCs) are a promising therapeutic target in cancer immunotherapy given their ability to prime antigen-specific T cells, and initiate antitumor immune response. A major obstacle for DC-based immunotherapy is the difficulty to obtain a sufficient number of functional DCs. Theoretically, this limitation can be overcome by using induced pluripotent stem cells (iPSCs); however, therapeutic strategies to engage iPSC-derived DCs (iPSC-DCs) into cancer immunotherapy remain to be elucidated. Accumulating evidence showing that induction of tumor-residing DCs enhances immunomodulatory effect of radiotherapy (RT) prompted us to investigate antitumor efficacy of combining intratumoral administration of iPSC-DCs with local RT.
Methods: Mouse iPSCs were differentiated to iPSC-DCs on OP9 stromal cells expressing the notch ligand delta-like 1 in the presence of granulocyte macrophage colony-stimulating factor. Phenotype and the capacities of iPSC-DCs to traffic tumor-draining lymph nodes (TdLNs) and prime antigen-specific T cells were evaluated by flow cytometry and imaging flow cytometry. Antitumor efficacy of intratumoral injection of iPSC-DCs and RT was tested in syngeneic orthotopic mouse tumor models resistant to anti-PD-1 ligand 1 (PD-L1) therapy.
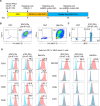
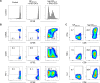
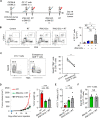
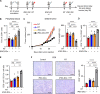
Results: Mouse iPSC-DCs phenotypically resembled conventional type 2 DCs, and had a capacity to promote activation, proliferation and effector differentiation of antigen-specific CD8+ T cells in the presence of the cognate antigen in vitro. Combination of in situ administration of iPSC-DCs and RT facilitated the priming of tumor-specific CD8+ T cells, and synergistically delayed the growth of not only the treated tumor but also the distant non-irradiated tumors. Mechanistically, RT enhanced trafficking of intratumorally injected iPSC-DCs to the TdLN, upregulated CD40 expression, and increased the frequency of DC/CD8+ T cell aggregates. Phenotypic analysis of tumor-infiltrating CD8+ T cells and myeloid cells revealed an increase of stem-like Slamf6+ TIM3- CD8+ T cells and PD-L1 expression in tumor-associated macrophages and DCs. Consequently, combined therapy rendered poorly immunogenic tumors responsive to anti-PD-L1 therapy along with the development of tumor-specific immunological memory.
Conclusions: Our findings illustrate the translational potential of iPSC-DCs, and identify the therapeutic efficacy of a combinatorial platform to engage them for overcoming resistance to anti-PD-L1 therapy in poorly immunogenic tumors.
Keywords: adaptive immunity; dendritic cells; programmed cell death 1 receptor; radioimmunotherapy; vaccination.
© Author(s) (or their employer(s)) 2021. Re-use permitted under CC BY-NC. No commercial re-use. See rights and permissions. Published by BMJ.
Conflict of interest statement
Competing interests: AO was a co-founder of Tactiva Therapeutics and receives research support from AstraZeneca and Tessaro.
Figures




Similar articles
-
Releasing the brakes of tumor immunity with anti-PD-L1 and pushing its accelerator with L19-IL2 cures poorly immunogenic tumors when combined with radiotherapy.J Immunother Cancer. 2021 Mar;9(3):e001764. doi: 10.1136/jitc-2020-001764. J Immunother Cancer. 2021. PMID: 33688020 Free PMC article.
-
IFNβ Is a Potent Adjuvant for Cancer Vaccination Strategies.Front Immunol. 2021 Sep 6;12:735133. doi: 10.3389/fimmu.2021.735133. eCollection 2021. Front Immunol. 2021. PMID: 34552594 Free PMC article.
-
Case Report: In Situ Vaccination by Autologous CD16+ Dendritic Cells and Anti-PD-L 1 Antibody Synergized With Radiotherapy To Boost T Cells-Mediated Antitumor Efficacy In A Psoriatic Patient With Cutaneous Squamous Cell Carcinoma.Front Immunol. 2021 Dec 23;12:752563. doi: 10.3389/fimmu.2021.752563. eCollection 2021. Front Immunol. 2021. PMID: 35003064 Free PMC article.
-
A Systematic Review of the Tumor-Infiltrating CD8+ T-Cells/PD-L1 Axis in High-Grade Glial Tumors: Toward Personalized Immuno-Oncology.Front Immunol. 2021 Sep 17;12:734956. doi: 10.3389/fimmu.2021.734956. eCollection 2021. Front Immunol. 2021. PMID: 34603316 Free PMC article.
-
Augmenting Anticancer Immunity Through Combined Targeting of Angiogenic and PD-1/PD-L1 Pathways: Challenges and Opportunities.Front Immunol. 2020 Nov 5;11:598877. doi: 10.3389/fimmu.2020.598877. eCollection 2020. Front Immunol. 2020. PMID: 33250900 Free PMC article. Review.
Cited by
-
Exploring the promising potential of induced pluripotent stem cells in cancer research and therapy.Mol Cancer. 2023 Nov 28;22(1):189. doi: 10.1186/s12943-023-01873-0. Mol Cancer. 2023. PMID: 38017433 Free PMC article. Review.
-
Intratumoral delivery of dendritic cells plus anti-HER2 therapy triggers both robust systemic antitumor immunity and complete regression in HER2 mammary carcinoma.J Immunother Cancer. 2022 Jun;10(6):e004841. doi: 10.1136/jitc-2022-004841. J Immunother Cancer. 2022. PMID: 35710296 Free PMC article.
-
Neoadjuvant In Situ Immunomodulation Enhances Systemic Antitumor Immunity against Highly Metastatic Tumors.Cancer Res. 2021 Dec 15;81(24):6183-6195. doi: 10.1158/0008-5472.CAN-21-0939. Epub 2021 Oct 19. Cancer Res. 2021. PMID: 34666993 Free PMC article.
-
Anti-PD-1 immunotherapy combined with stereotactic body radiation therapy and GM-CSF for the treatment of advanced malignant PEComa: A case report.Front Oncol. 2023 Apr 18;13:1045119. doi: 10.3389/fonc.2023.1045119. eCollection 2023. Front Oncol. 2023. PMID: 37143946 Free PMC article.
-
PD-L1+ macrophages suppress T cell-mediated anticancer immunity.Oncoimmunology. 2024 Apr 4;13(1):2338951. doi: 10.1080/2162402X.2024.2338951. eCollection 2024. Oncoimmunology. 2024. PMID: 38590800 Free PMC article.
References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
